The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
Birds are now considered “living dinosaurs” by evolutionary theorists. The putative descent of birds from dinosaurs has become established as one of the most popular evolutionary transitions. In recent years, apparent bird-like features have been increasingly detected in theropod dinosaurs, and a majority of apparent avian traits have been found to be represented in various dinosaur groups. Therefore, a gradual transition from dinosaurs to birds is considered by evolutionary theorists well supported by fossil evidence. Only a few characteristics are considered suitable to distinguish between birds and dinosaurs. Thus, one could no longer draw a line between dinosaurs and birds today according to evolutionists. The occurrence of a number of bird-typical traits in dinosaurs and Mesozoic birds is here analyzed in more detail. In which theropod groups do the traits in question occur, and what is the phylogenetic and stratigraphic position of the genera in question? Is it supported by data that the apparent avian features were added step by step? The cladistic methodology is used to test whether consistent evolutionary hypotheses can be made within an evolutionary interpretive framework in light of the fossil evidence. The following traits or trait complexes are examined: feather types and flight ability, teeth and beak, brain and encephalization quotient, furcula, gastralia, rib cage, sternum, pneumaticity, air sac system and respiration, pelvis and posteriorly oriented pubis, pygostyle, fibula, wrist, and eggs, clutch, and brood care. It was concluded that even after examining the claimed fossil evidence, there are no unequivocal evolutionary transitions. Instead, the evidence confirms the creation of a variety of original basic types (created kinds) that subsequently diversified.
Summary of Findings
Assuming an evolutionary emergence the resulting analysis shows that a number of apparent bird-typical traits that occur in theropod dinosaurs would have evolved multiple times convergently and were presumably not expressed at all in phylogenies at the base of the respective groups. For almost all studied traits, convergences have to be assumed more or less frequently due to the mosaic-like distributions. In some cases, different relationships are suggested depending on the underlying trait. This manifests itself, for example, in the fact that a number of genera, measured against established phylogenies, show contradictory trait combinations that make phylogenetic classification more or less difficult. This often mosaic distribution also favors the controversial interpretation that some genera interpreted as avian precursors may be secondarily flightless birds. In this case, their features would be eliminated as evidence for a gradual transition from dinosaurs to birds. Some features continue to show significant jumps.
Based on these findings, the claim of a gradual evolutionary “buildup” of the avian assemblage based on the fossil record is strongly questioned and is not supported by the fossil record for some traits. Moreover, these and other commonly encountered findings do not conform to evolutionary theory expectations and may be considered anomalies:
- Based on the distributions of features in early birds and the dinosaurs close to them and their stratigraphic positions, a picture emerges of different mosaics and consequently of a network of similarity relationships.
- It is not clear which group is at the base of the birds.
- The large extent of homoplasy means that the cladograms are not stable and new finds can lead to major changes.
- The oldest bird groups are already strongly differentiated at the base and there are partly also “modern” characteristics in the oldest representatives of a group.
- Especially in plumage characteristics, the greatest degree of diversity is seen near the base of the bird groups.
- Most theropod dinosaur genera that have apparent bird-like features are geologically younger than the geologically oldest birds.
Some findings appear able to be interpreted well from an evolutionary point of view (which does not mean that this interpretation is correct). Some (but by far not all) mosaic forms could possibly be close to evolutionary transitional forms. In some groups, apparent trends in the change of trait expression can be traced over the course of the Cretaceous. Also, the fact that many apparent bird features appear to occur in different dinosaur groups of theropods could be interpreted to be evolutionary with certain restrictions (for example, convergence problems).
“Evolutionary Experiments”
The mosaic character of the trait distributions and especially the diversity of early established feather types and modes of flight (with four-winged forms and partly unclear flight capabilities) leads many researchers to assume a kind of “evolutionary experimentation.” This terminology, however, is questionable in evolutionary hypotheses because it implies a goal orientation, which, however, is not inherent in a natural process. The talk of experimental phases in evolution conceals the evolutionary-theoretical problem of a rapidly established variety of different mosaics.
Creation
In the questions on origins in biology usually only explanations are discussed which are committed to the naturalistic paradigm. For this determination there is neither a scientific nor a philosophical justification, but it is a convention, which can also be discarded. Under the assumption of the creation of flexible, adaptable basic types (created kinds), the findings that are problematic in terms of evolutionary hypotheses can be explained:
- The explosive occurrence of the variety of forms reflects the existence of basic types (created kinds), which were created in finished distinct and diverse form.
- The numerous, most diverse mosaic forms are the expression of manifold combinations of characteristics, the expression of which is explained by the respective way of life and not by a preceding evolution.
- The difficulties that arise for evolutionary theoretical modeling of how the various mosaic forms arose become superfluous if the traits can be freely combined according to the requirements for particular lifestyles and habitats.
- The discussed problem of an “experimentation” is omitted. There are no “experiments,” but an initial variety of forms, which was originally in some respects the greatest compared to today’s diversity (especially with feather types and flight forms).
Introduction
It is now claimed: “Birds are living dinosaurs” (Erickson et al. 2017), and “In truth, birds are dinosaurs” (Brusatte 2017b, 531). One may find this equation of birds and dinosaurs odd or even outlandish, but in recent decades the putative descent of birds from dinosaurs has become established as one of the most popular evolutionary transitions, at least according to the conviction of the majority of paleontologists (for example, Brusatte, O’Connor and Jarvis 2015, R8882). This claimed transition is defended against criticism almost as vigorously as the underlying evolutionary paradigm itself. The basis for determining the ancestral relationship here is trait comparisons of different taxa. The fact that birds are even identified as “dinosaurs” has to do with the method of cladism, which is also used almost unchallenged in evolutionary research. According to this, the system of living beings and thus the presumed ancestral relationships are brought into a bifurcated (cladistic) system on the basis of so-called derived (“advanced”) characteristics, in which birds represent a branch in the “dinosaur tree”—hence the paradoxical-sounding statement that birds are not only descended from dinosaurs, but are even dinosaurs. Accordingly, many dinosaur genera that are placed close to birds are called “non-avian dinosaurs” (see table 1 for this term). Fig. 1 shows the most important groups of the assumed evolutionary transitional range of dinosaurs and birds in a cladogram.3
Table 1. Evolutionary hypothesis-laden terms and phrases and their translation into theory-free form.
| Gradual evolution | Genera with an increasing number of bird-typical features have survived in geologically increasingly younger dated strata |
|---|---|
| Cretaceous birds | Birds that are recorded in the fossil record only in the Cretaceous geological system. |
| early birds | the geologically oldest birds |
| basal species | Species that are placed at the base of cladograms based on their traits or trait expressions according to cladistic analyses. |
| original (plesiomorphic) | also “primitive”. Traits interpreted as initial in the presumed evolution (standing at the base). |
| derived (apomorphic) | also “progressive”. Traits interpreted as occurring late in the presumed evolution (deeply nested). |
| already formed | Formulation expressing that a feature is geologically established early in evolutionary theory interpretation. |
| experimental phase | Assumption of an early evolutionary phase in which no clear evolutionary lines are yet discernible. |
| Non-avian dinosaurs | Dinosaurs placed in groups close to birds in cladograms. The term suggests that birds are evolved dinosaurs. |
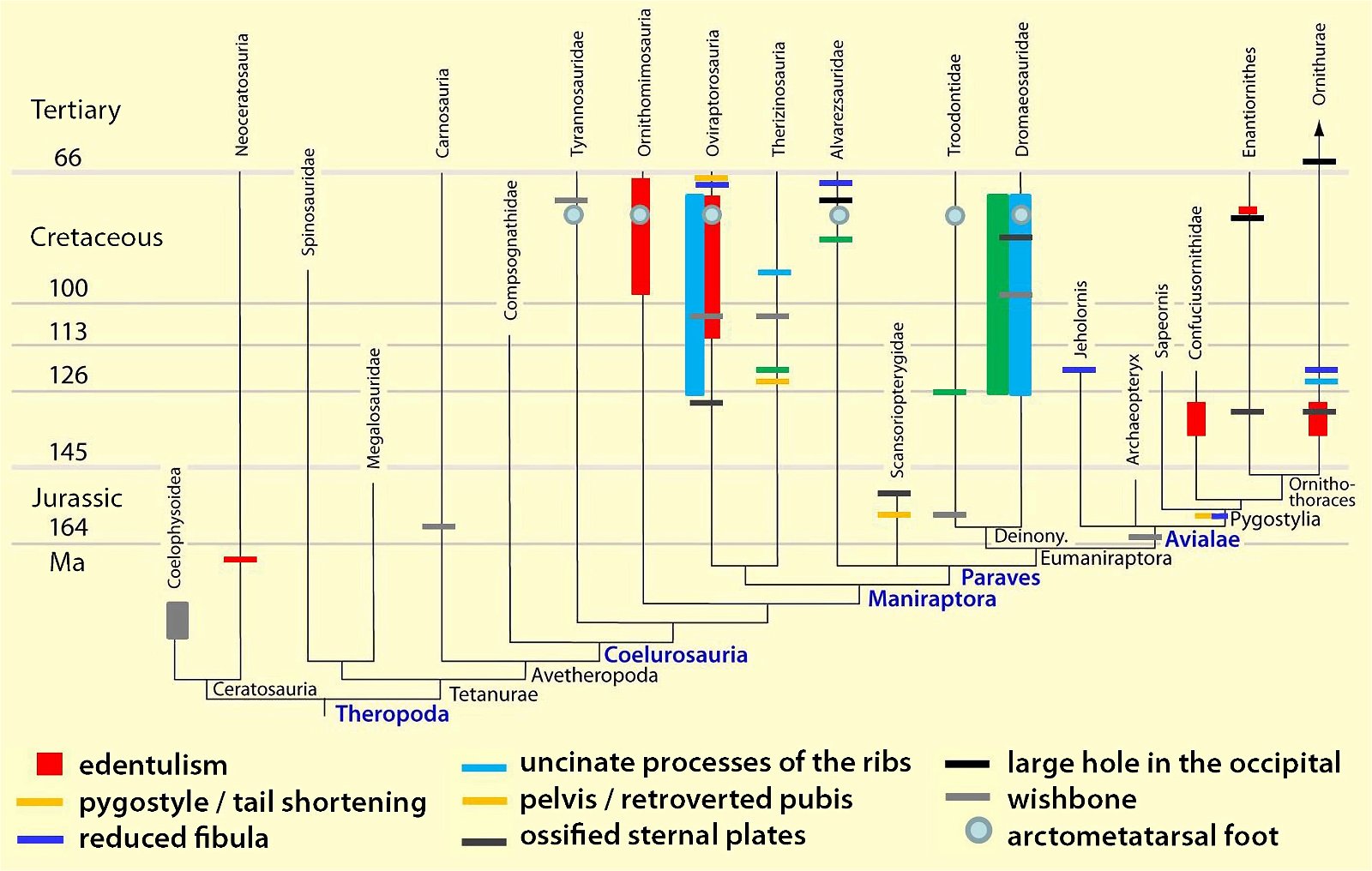
Fig. 1. Time-scaled cladogram of theropod-dinosaur groups and birds and synopsis of the convergent distribution of some bird-typical features discussed below. The phylogenetic reconstructions of different workers often yield different arrangements of individual groups. However, the various cladogram variants do not substantially change the multiple convergence. In many cases the distributions of features are so unsystematic that the assumption of numerous convergences is unavoidable. Ma: million radiometric years (according radiometric dating which is not the same as real years). (Assembled from numerous sources.)
In recent years, a number of studies have been published that purport to document a gradual transition from dinosaurs to birds. The main message is: The features that are characteristic of birds were essentially already formed in different frequencies in different dinosaur genera from several families of theropods. Therefore, it is no longer possible to draw a line between dinosaurs and birds today (Brusatte 2017b, 554). Thus, numerous “non-avian dinosaur” genera are known to possess apparent bird-like characteristics. Foremost among these features are feathers or feather-like body appendages5, the wishbone (furcula), a large sternum, ossified sternal plates, an enlarged encephalization quotient, long arms, the construction of the wrist (with a semilunate carpal in the carpus6), a three-fingered hand with a long second finger7, pneumaticity, the air sac system, hooked, posteriorly directed projections on the anterior ribs8, a posteriorly directed pubis, bipedal locomotion, long hind legs with a three-toed foot, a reduced fibula, fused caudal vertebrae, brood care, a significantly reduced body size, and others (Organ et al. 2007; Padian and Chiappe 1998a, 44; Xu 2006; Xu et al. 2014; see fig. 2).
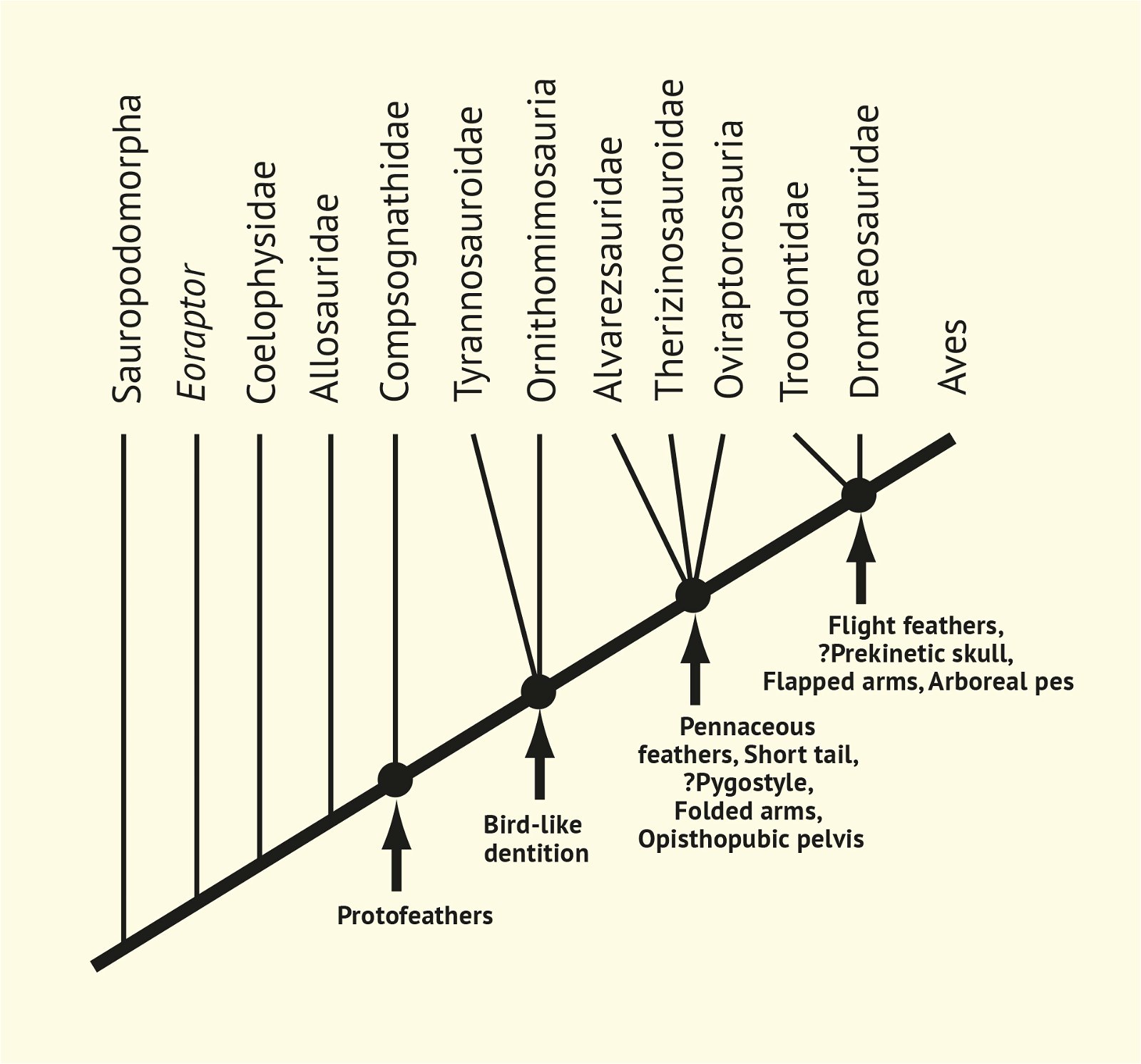
Fig. 2. Stepwise acquisition of bird traits according to Xu (2006).
Conversely, many Upper Jurassic (most notably Archaeopteryx) and Lower Cretaceous birds possess features that are atypical of present-day birds but seem to be developed in many theropod genera. These include the possession of teeth (widespread, with varying degrees of reduction of teeth in different genera), a long caudal spine (in Archaeopteryx and Jeholornis, instead of a short pygostyle), lack of a sternal keel, relatively few bone fusions, and other features.
Seen in this light, it seems well founded that there is an almost continuous gradual transition from theropod dinosaurs to birds. This assessment is supported by the fact that within the individual relevant features there are also still different expressions, for example, a different extent of reduction of teeth or differently expressed pygostyles (for details see below).
But, even when we closely examine the claimed fossil evidence from the evolutionists’ perspective, from their own assessments of the fossils it can be shown that this picture is incomplete and more or less misleading for the following reasons:
- Many apparent bird features in dinosaurs occur several times independently (convergent), that is, not only in a single, but in different lineages without a supposedly common ancestor. Moreover, the individual apparent bird features are partially distributed on different branches of theropods (fig. 1).
- The stratigraphic positions of the dinosaur genera, which possess a different number of apparent bird-typical features, do not correspond in many cases to the evolutionary sequences to be assumed (see figs. 3–5). The increase of apparent bird-typical features is only a rough tendency when considering several lineages at the same time.
- The same applies to dinosaur features and their successions in Upper Jurassic and Lower Cretaceous birds.
- Some genera, which are interpreted as bird precursors, could be secondarily flightless birds. Their traits would in this case be eliminated as evidence for an assumed gradual transition from dinosaurs to birds.
- In some features there are more or less prominent gaps (where gradual reconstruction would be functionally prohibitive).
- A number of genera have contradictory combinations of traits that make phylogenetic classification more or less difficult.
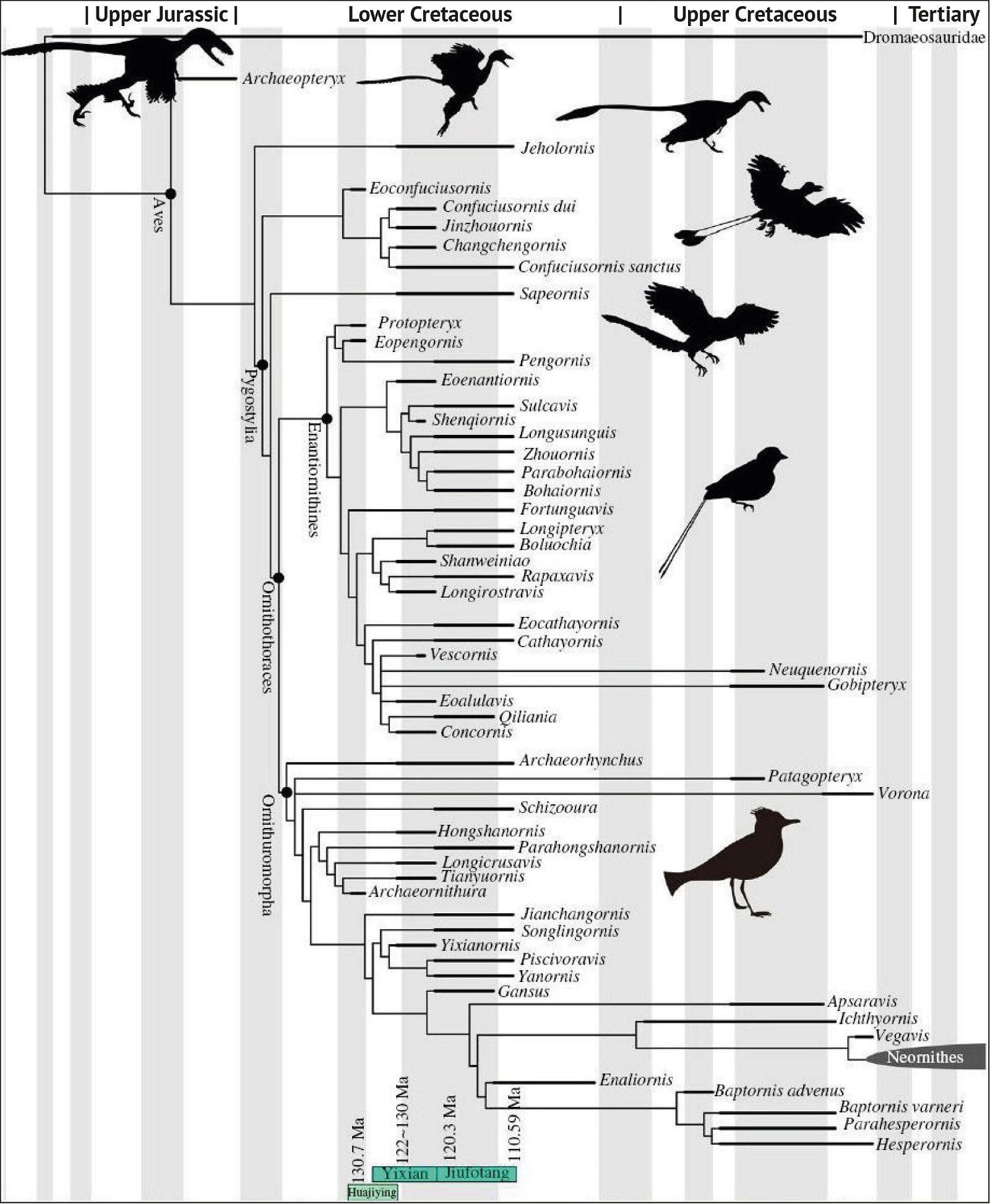
Fig. 3. Detailed time-scaled phylogeny of Mesozoic birds after Wang and Lloyd (2016). The cladogram is the consensus tree obtained from the phylogenetic analysis. The thicker lines represent the dating of the upper and lower limits of the geological strata in which the genera of interest were found. (© 2016 National Academy of Sciences)
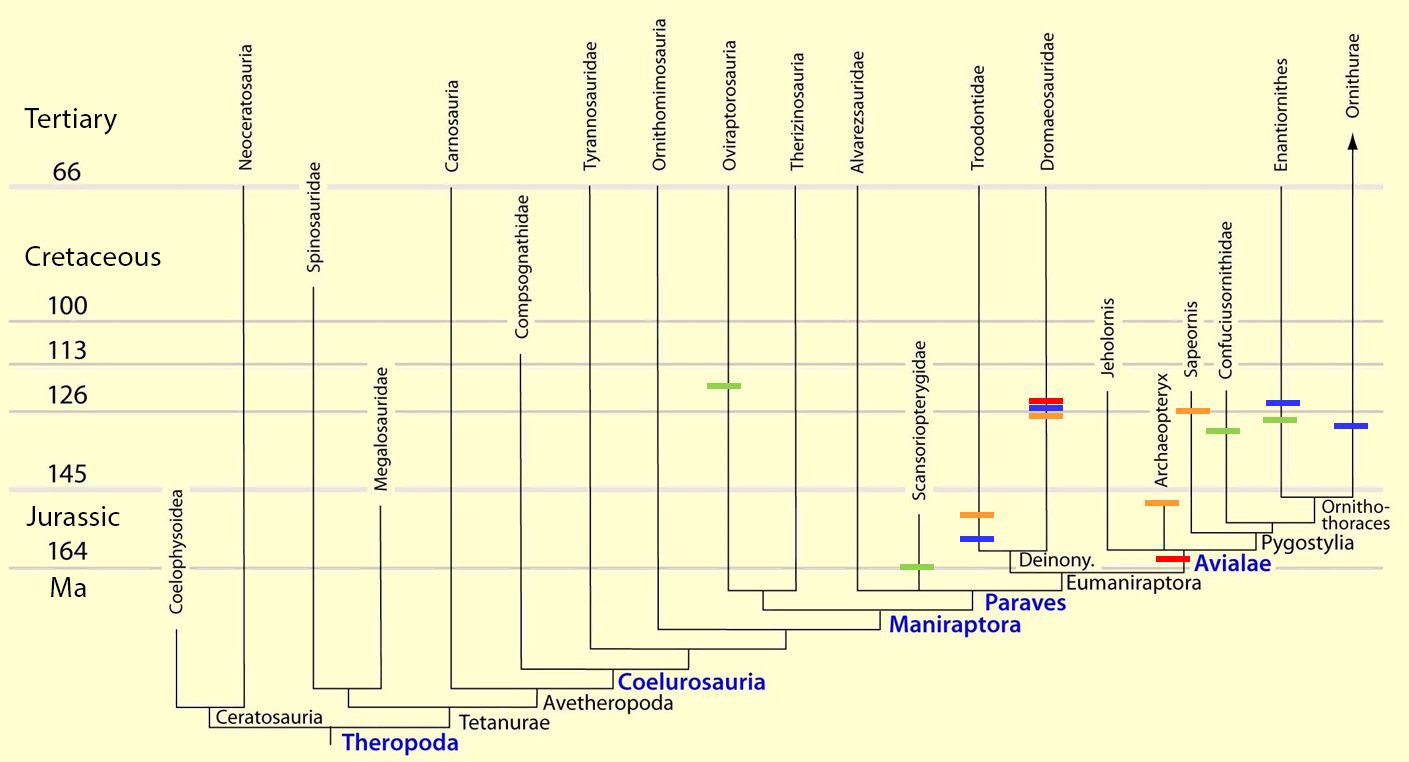
Fig. 4. Time-scaled phylogeny of theropod dinosaur groups and birds. The red bars represent the upper and lower limits of the geological strata in which the groups in question were found. (Assembled from numerous sources; see also the note at fig. 1)
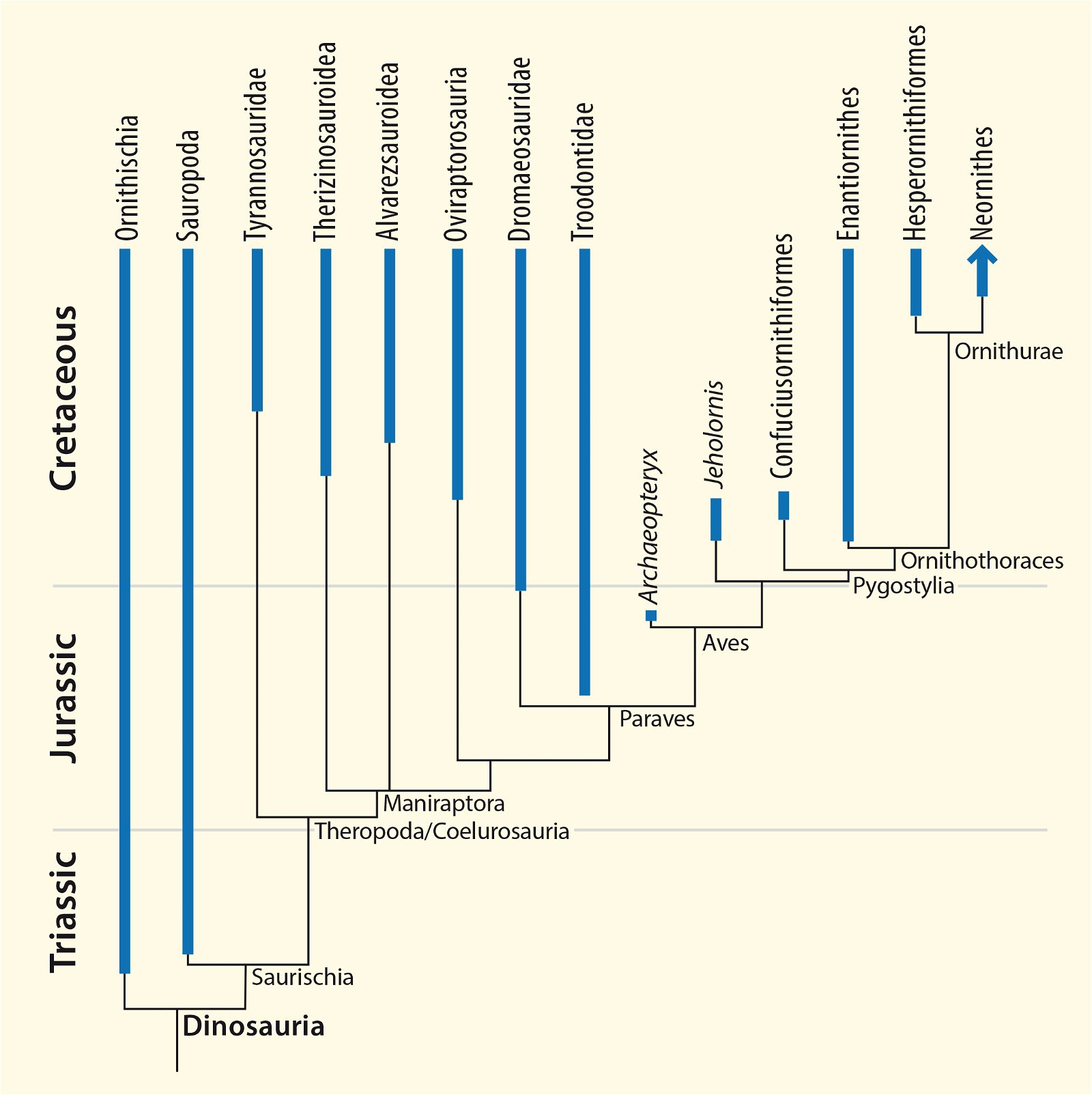
Fig. 5. Time-scaled phylogeny of some dinosaur groups and birds after Varricchio and Jackson (2016).
This situation manifests itself, for example, in the fact that no specific genus can be given as the (ultimate) ancestor of birds: “Although virtually all recent analyses put Dromaeosauridae or Troodontidae (or the two together as Deinonychosauria) as the sister group of Aves, neither is truly the ancestor, and hence known forms like Deinonychus or Troodon can only go so far as models for the true avian ancestor” (Witmer 2002, 16).
The points mentioned will be elaborated on in more detail below and supported by numerous examples.
Quotes on Gradual Acquisition of Bird Characteristics
In the following, some quotations from evolutionary theorists are gathered together, according to which bird-typical characteristics had gradually developed in dinosaurs already before the origination of birds. One of the main goals of this paper is to challenge these claims based on the fossil record.
- “In summary, a great many skeletal features that were once thought of as uniquely avian innovations—such as light, hollow bones, long arms, three-fingered hands with a long second finger, a wishbone, a backward-pointing pelvis, and long hind limbs with a three-toed foot—were already present in theropods before the evolution of birds. Those features generally served different uses than they did in birds and were only later co-opted for flight and other characteristically avian functions, eventually including life in the trees.” (Padian and Chiappe 1998a, 44)
- “Preliminary analysis of character evolution suggests that the major avian osteological characters were acquired during the early evolution of maniraptoran dinosaurs.” (Xu 2006, 4)
- “However, nearly every single character that at one time was thought to make something a ‘bird’ is now known to occur progressively earlier in theropod evolution. Therefore, ‘bird’ is a colloquial term that lacks a meaningful taxonomic or scientific basis as it has no precise phylogenetic meaning.” (Turner, Makovicky, and Norell 2012, 14)
- “When placed together on a family tree, these fossils show that many anatomical components of the modern-bird flight apparatus evolved piecemeal over tens of millions of years of dinosaur evolution, for reasons unrelated to flight.” (Brusatte 2017a, 793)
- “Indeed, if we look at theropod history across the whole of the Triassic, Jurassic and Cretaceous, we see a gradual, cumulative acquisition of bird-like features, ranging from wishbones and a pneumatised skeleton to complex feathers, a reduced, three-fingered hand, an enlarged sternum (breastbone) and tiny size. . . . a robust and well-supported model showing a prolonged, directional trend in size reduction in the theropod lineage leading to birds: a trend that is continuous across 50 million years of theropod history, and which shows the animals at successive nodes becoming ever-smaller as we get closer to birds in the phylogeny. . . . Then there’s the fact that, as we get closer to birds in the phylogenetic tree, we see an increasingly elaborate plumage, a more bird-like system of body and hindlimb orientation linked to a shift in the center of gravity, a stiffer, slimmer tail, and a number of behaviors that involve a degree of climbing (Birn-Jeffrey et al. 2012) and gliding (Dyke et al. 2013).” (Naish 2014)
- “most of the 30 or more characteristics that distinguished the small, flying Archaeopteryx from ground-dwelling, carnivorous dinosaurs (theropods) emerged much earlier.” (Benton 2014, 508)
- “Birds evolved significantly faster than other theropods, but they are indistinguishable from their closest relatives in morphospace. Our results demonstrate that the rise of birds was a complex process: birds are a continuum of millions of years of theropod evolution, and there was no great jump between nonbirds and birds in morphospace, but once the avian body plan was gradually assembled, birds experienced an early burst of rapid anatomical evolution.” (Brusatte et al. 2014, 2386)
- “In general anatomical terms, birds are a continuum of millions of years of theropod evolution. There is no great jump between nonbirds and birds in morphospace. Instead, those features that today combine to set birds apart from other vertebrates-feathers, wishbones, air sacs, and hundreds more-evolved piecemeal in Mesozoic theropods,” (Brusatte et al. 2014, 2389)
- “What was once seen as a rapid adaptive radiation, in which Archaeopteryx rapidly acquired 30 or more avian apomorphies, is now seen as a stepwise process of more than 50 million years.” (Puttick, Thomas, and Benton 2014, 1497)
- “Recent discoveries of spectacular dinosaur fossils . . . demonstrate that distinctive bird characteristics such as feathers, flight, endothermic physiology, unique strategies for reproduction and growth, and a novel pulmonary system originated among Mesozoic terrestrial dinosaurs. . . . The iconic features of extant birds for the most part evolved in a gradual and stepwise fashion throughout archosaur evolution.” (Xu et al. 2014)
- “Thus, there is no sharp line demarcating bird and nonbird – the distinction has become entirely arbitrary.” (Witmer 2002, 6)
- “Currently, Aves is without a character-based definition; the last notable attempt—more than half a century ago—employed three skeletal features (the presence of a furcula, retroverted pubes, and a reversed hallux) and the presence of feathers (de Beer 1954). However, these features no longer define Aves, being either present in non-avian dinosaurs (furcula, feathers) or absent in basal-most birds (retroverted pubes, reversed hallux)” (O’Connor and Zhou 2015, 334). However, the authors see possibilities for a biological definition of the birds: possession of a crop and loss of the right ovary.
- “many features that are commonly associated with birds, flight, and arboreal life, such as the thin-walled bones, the furcula, the long forelimbs, the sideways-flexing wrist, and feathers, evolved in animals other than birds and for purposes other than flight; they were later exapted for other functions.” (De Ricqlès et al. 2003, 373)
- “many of the traits that are considered uniquely avian among extant amniotes actually arose before the origin of birds themselves.” (Makovicky and Zanno 2011, 10)
- “The fact that scientists are having a difficult time distinguishing the earliest birds from their closest dinosaur relatives illustrates just how bird-like some non-bird dinosaurs were (. . .), and how the transition between non-bird dinosaurs and birds was gradual.” (Brusatte, O’Connor, and Jarvis 2015, 889)
Methodological Preliminary Remarks
The argumentation of the following analyses takes place within the framework of the evolutionary paradigm, which, however, is certainly not regarded as fixed by the author. This approach is taken to demonstrate that even with the evolutionary paradigm the fossil evidence is inconclusive and does not reveal a graduated series of transitions. However, the evolutionary paradigm is so firmly established in research that often no (longer) clear distinction is made between data and interpretations, and presumably a sensitivity to this is largely lacking. Phrases such as “gradual emergence,” “Cretaceous birds,” “early birds,” “basal species,” “original” (plesiomorphic), “derived” (apomorphic); “already formed,” “experimental phase,” and others are evolutionary theory-laden. It would be impractical to constantly challenge these terminologies, so they are used in part but are intended to be understood in a descriptive sense. For example, “early birds” are those found in geologic sediments that are assigned a relatively old age in the system of historical geology. “Early” here, however, is not meant to imply that an early phase of hypothetical evolution is involved (see table 1 for other evolutionary theory-laden terms).
On the point of the theory-loadedness, it is driven by today’s usual designation “non-avian dinosaurs.”9 That term is not used here, but they are instead referred to as “theropod dinosaurs.” In this paper, birds are not referred to as dinosaurs.
Again, the aim now is to closely examine the claimed fossil evidence from the evolutionists’ perspective and establish that from their own assessments of the fossils that their picture of an evolutionary transition of dinosaurs to birds is totally incomplete and more or less misleading, so that birds simply cannot be designated as “living dinosaurs.” The paradigmatic framework of general evolution is abandoned when later the findings discussed are interpreted within the framework of a creation paradigm.
Bird-like Features in Theropods: Antecedents or Convergences?
For a number of important bird-like features in genera placed among dinosaurs, what is known about their distribution in different genera will be examined. The background is the claim, documented in the introductory section, that most bird traits evolved step by step in dinosaur lineages. Can this be verified on the basis of trait distribution? And do the stratigraphic positions of those genera interpreted as precursors fit the phylogenetic reconstructions?
For some traits, we simultaneously investigate the extent to which dinosaur-typical or bird-untypical traits gradually decline in birds. Thus, this comprehensive section is concerned with the first three of the abovementioned topics. Not all traits are treated, but a larger representative selection of traits that are typical for today’s birds or which sufficiently informative data material could be compiled.
Feather Types and Flight Capability
In a large number of theropod genera which are systematically placed in proximity to birds hair-like or feather-like appendages or even true pennaceous feathers have been fossilized. In the evolutionary literature, a wide variety of body appendages are referred to as “feathers.” In many cases, this designation is motivated by evolutionary theory and not by morphological findings, namely in all cases where they are hairy, downy, or bristle-like appendages. For the sake of simplicity, we will nevertheless refer to “feathers” in this broad sense in the following. The fossil forms known in the meantime are even supposed to prove a rather gapless succession of different stages from simple body appendages to flat bird feathers. However, the transitions are by no means smooth. There are clear differences between hair-like, downy (possibly branched), or bristle-like appendages on the one hand, and flat, flight feathers on the other (overview and source evidence in Junker 2017, see fig. 6). There is circumstantial evidence that some pennaceous feathers are to be interpreted as regressions. The forms in question would thus be flightless descendants of birds (more in Junker 2017).
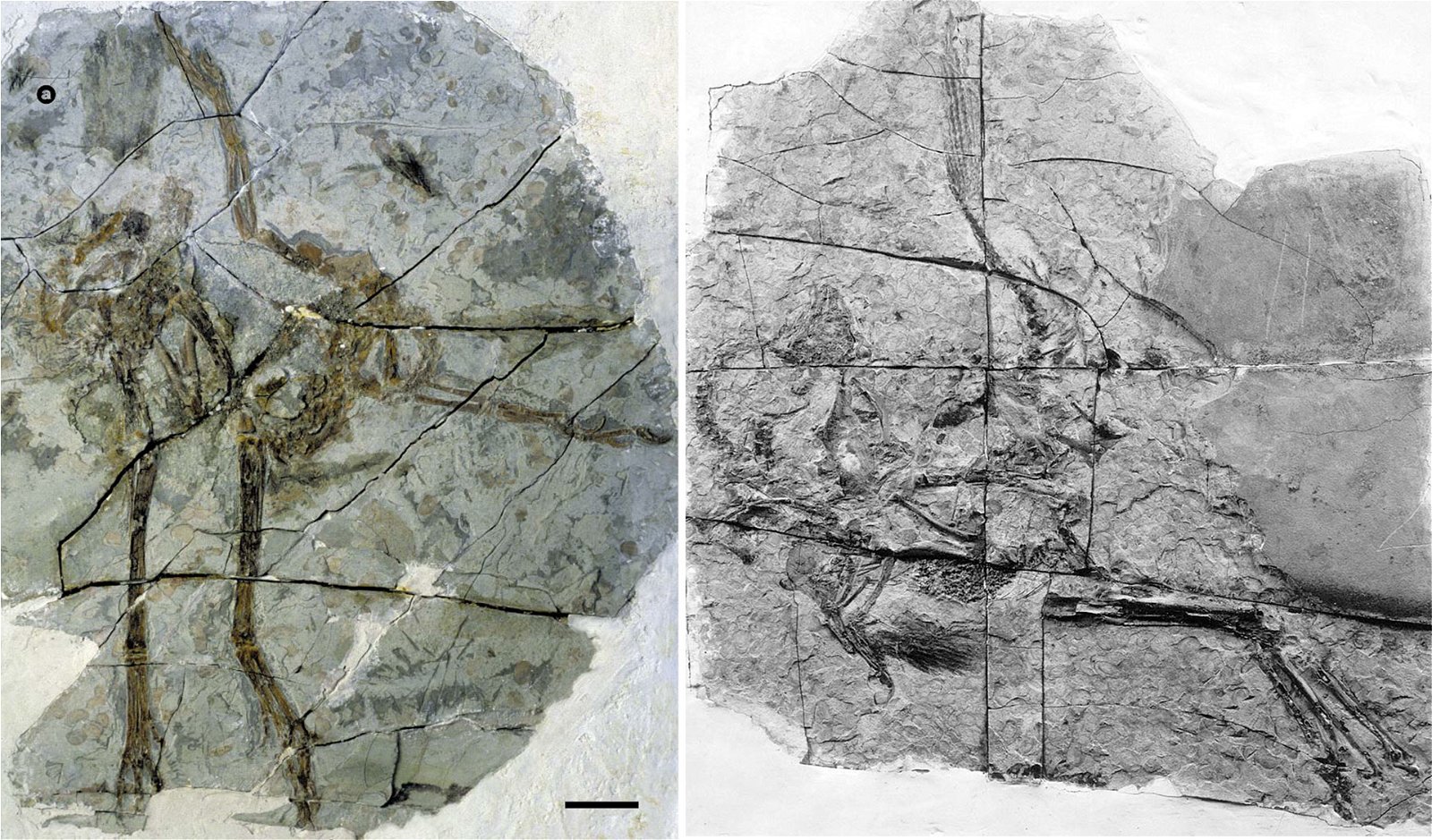
Fig. 6. Oviraptorid genera with symmetrical planar feathers: Left: Protarchaeopteryx robusta, holotype Jonathan Chen, “Holotype of Protarchaeopteryx robusta on display at the Geological Museum of China,” https://commons.wikimedia.org/wiki/File:Protarchaeopteryx-Geological_Museum_of_China.jpg, CC BY-SA 4.0. Right: Caudipteryx zoui, holotype, Gareth J. Dyke and Mark A. Norell, “Photograph of the holotype specimen of Caudipteryx zoui (NGMC 97−4−A) described by Ji et al. (1998),” CC BY 2.0). In evolutionary theory interpretation, there is some evidence that feather symmetry is secondary (see Junker 2017).
According to Brusatte (2017a, 792), the evolution of flight ability was “chaotic.” Different dinosaurs would have “experimented” with different airborne behaviors and different feather arrangements until finally only modern birds survived.10 Apart from the fact that the term “experimenting” is highly problematic in evolutionary theory (see later), it is clear from this quotation that there is no question of a linear, step-by-step development. Rather, in evolutionary theoretical reading, different expressions of flight ability are found in different lineages, which at least in part cannot be brought into an evolutionary sequence. For example, four-winged forms such as the dromaeosaurid Microraptor are thought to represent a distinct extinct lineage that cannot be interpreted as precursors to two-winged forms. This is also true for the species Yi qi (Xu et al. 2015) and Ambopteryx longibrachium (Wang et al. 2019) from the family Scansoriopterygidae, which possessed a distinct flying skin and cannot be conclusively placed in a lineage relationship with other Paraves.
In addition, Dececchi, Larsson, and Habib (2016) demonstrated through a biomechanical study that for behaviors supported by fluttering or wing-beating (wing-assisted incline-running, flap running, wing-assisted leaping), there is no discernible continuous trend of refinement in biomechanical performance with respect to these behaviors using the phylogenetic successions of theropod dinosaurs.11 Some species of Paraves probably could fly or glide, but others very likely could do neither. Based on the many morphological differences among winged Paraves and birds that have survived in the Upper Jurassic and Lower Cretaceous, it follows evolutionarily that active flight arose independently not just once, but in many different groups (Brusatte 2017a, 793; Wang et al. 2019).12 Not long ago, the possibility of multiple independent origins of active bird flight would have been ruled out evolutionarily. Foth, Rauhut and Tischlinger state (2015, 28): “New phylogenetic analyses of predatory dinosaurs (theropods) show: Wings with asymmetric flight feathers arose several times during evolution.” And further (page 33): “However, we suspect that such hand wings evolved separately several times, because other dromaeosaurs (Sinornithosaurus) and early representatives of the avian lineage (Anchiornis) still possessed the original symmetrical pennaceous feathers on the arms. Based on the better aerodynamic properties of the asymmetric feather type, it can further be assumed that consequently also the flight ability within Pennaraptora evolved independently several times, at least twice—an important new finding” (see also Foth and Rauhut 2017; see below). The discovery of Ambopteryx (Wang et al. 2019) has added another independent lineage of flight-capable forms from an evolutionary theoretical perspective (or its existence has been confirmed13).
In addition, just at the beginning of the presumed evolution of birds, a great variety of feather types have been fossilized, including those that are not known otherwise and among birds today. This is true, for example, of the troodontid Anchiornis, which possessed, among other things, a feather type that was neither typically down-like nor exhibited in its shape typical characteristics of a pennaceous feather, nor was it intermediate between these two feather types (Saitta, Gelernter, and Vinther 2017, fig. 7). Moreover, the entire plumage of Anchiornis was as unique as that of the recently discovered genus Serikornis (Lefèvre et al. 2017; fig. 8) from the same family with, again, a different mosaic of characters regarding feathering that is difficult to classify in evolutionary theory.14
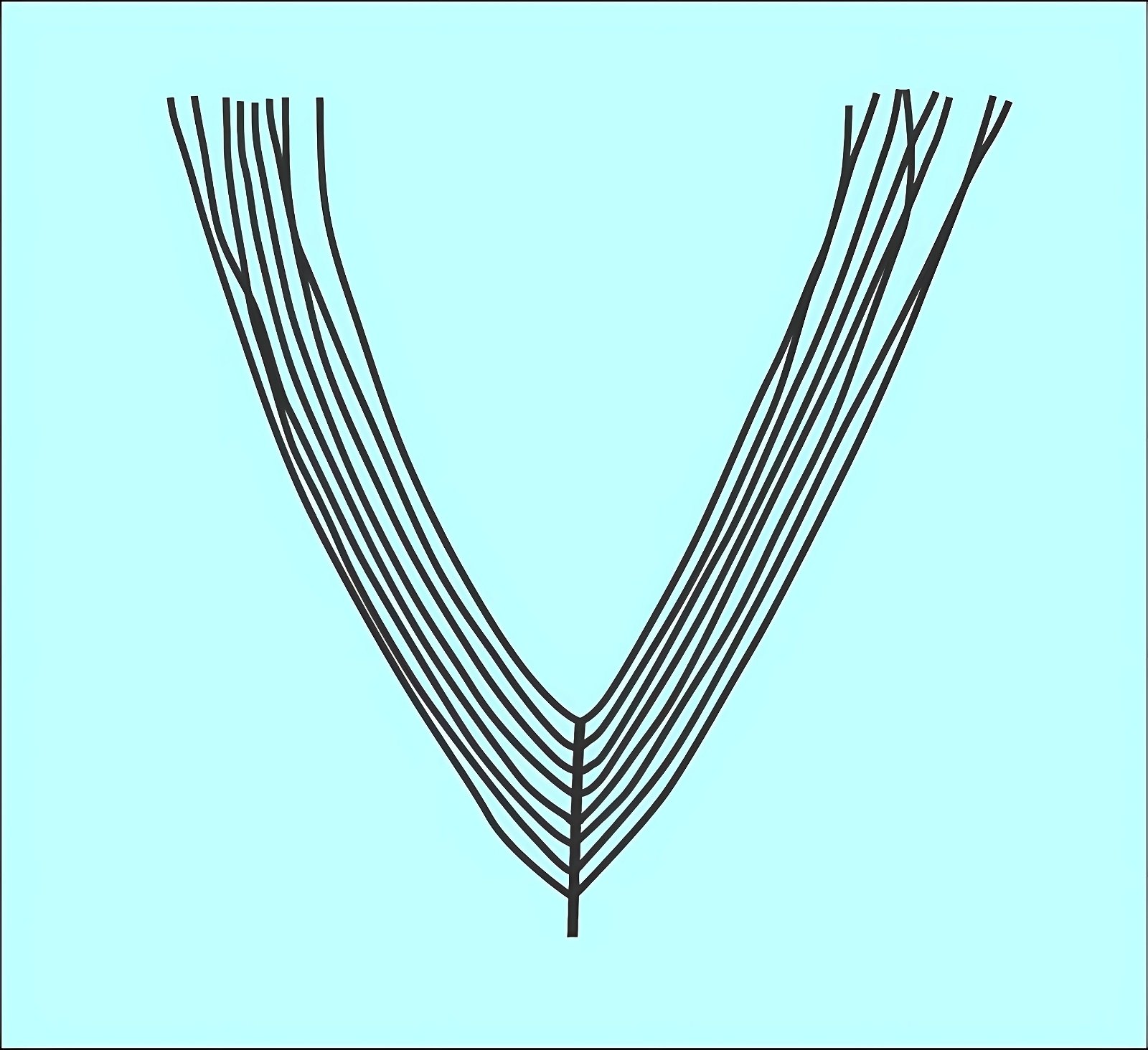
Fig. 7. Newly discovered feather type in Anchiornis known only from fossils (after Saitta, Gelernter, and Vinther 2017).

Fig. 8. Reconstruction of Serikornis. This genus possessed both downy tufted and simple pinnate body appendages. Nevertheless, the interpretation as an intermediate form transitioning to birds is problematic. For more details, see Junker (2017). Emily Willoughby. “Life restoration of Serikornis sungei, feathered Paraves|paravian dinosaur from the Upper Jurassic Tiaojishan Formation of Liaoning, China, described in 2017.” https://en.m.wikipedia.org/wiki/File:Serikornis.jpg, (CC BY-SA 4.0).
Furthermore, ribbon-like pennaceous feathers were discovered in the Upper Jurassic genus Epidexipteryx (fig. 9). Evolutionary theory suggests that this feather type evolved independently at least four times (Xu, Zheng, and You 2010): not only in Epidexipteryx, but also in the Confuciusornithids, in some opposite birds (such as one of their oldest genera, Protopteryx), and in the oviraptorosaurid Similicaudipteryx (Prum 2010). In Protopteryx, the tail feathers were unusual and uniquely developed. They were unbranched in the region near the body (Zhang and Zhou 2000, 195715). Other feather types include elongate broad filamentous feathers in the therizinosaur Beipiaosaurus (Xu, Zheng, and You 2009, fig. 10) and a previously unknown expression in the Lower Cretaceous genus Cruralispennia from the enantiornithine group. Their feathers were wire-like in the proximal region and had distally filamentous tips (“proximally wire-like part with a short filamentous distal tip;” Wang et al. 2017d; fig. 11). A greater diversity of feather types than today was established early16 and occurred quite abruptly. And it does not fit easily into an evolutionary theoretical scheme (see below).

Fig. 9. Reconstruction of Epidexipteryx. (Photo: LWL Museum of Natural History, Münster, Germany).
Fig. 10. Reconstruction of Beipiaosaurus inexpectus. Matt Martyniuk, “Life restoration of the therizinosaur Beipiaosaurus inexpectus, based on skeletal reconstruction by Jaime Headden and feathers as preserved in the holotype and referred specimens,” https://commons.wikimedia.org/wiki/File:Beipiao1mmartyniuk.png, CC BY-SA 3.0.

Fig. 11. Unusual shape of some feathers on the legs of Cruralispennia. More details in the text. Scale bar: 10 mm. (From Wang, Li, and Zhou 2017. CC SA 4.0)
Long feathers on the leg are also said to have been acquired independently several times. The anchiornithid genera Xiaotingia, Pedopenna, and Anchiornis possess long feathers on the midfoot. “However, this particular feature was apparently developed at least twice more in parallel by Microraptor and Sapeornis” (Moser 2014, 416f.). Leg feathers have also recently been demonstrated in Archaeopteryx (Foth, Tischlinger, and Rauhut 2014). Sullivan, Xu, and O’Connor (2017, 13) calculate that leg feathers evolved independently four times (Anchiornis, Archaeopteryx, some dromaeosaurids, possibly Sapeornis).
The Lower Cretaceous enantiornithine Schizooura possessed unusual forked tail feathers, which is uncommon among Lower Cretaceous enantiornithine birds where only fan-shaped tails are otherwise known. Zhou, Zhou, and O’Connor (2012) note in this regard that this tail feather morphology would reduce aerodynamic efficiency in modern birds compared to the fan-shaped tail, but may have played a role in courtship.
Sullivan, Xu, and O’Connor (2017) conclude from the available evidence on the feathering of Paraves and early birds that rapid diversification of aerodynamic structures is conceivable. Either flight ability must have been a primitive trait in Paraves and lost multiple times, or it must have been acquired independently multiple times (fig. 12). And if flight ability was an original trait among Paraves and Aves, it must have been extensively transformed in different lineages (Sullivan, Xu, and O’Connor 2017, 12). Further, they conclude that the reduction of tail feathering to a small number of tail feathers occurred independently in Confuciusornithidae and Enantiornithes (Sullivan, Xu, and O’Connor 2017, 9).
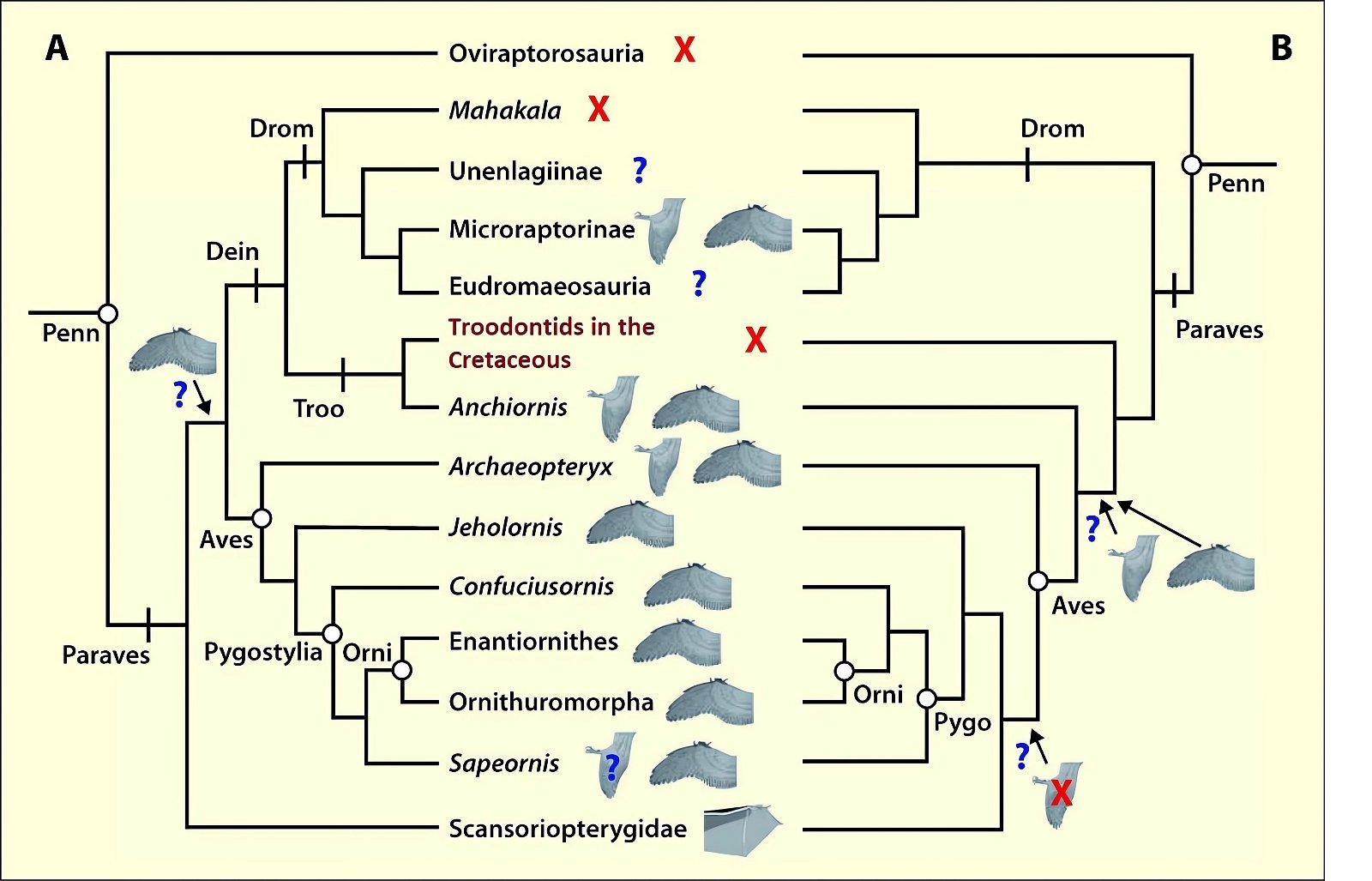
Fig. 12. Distribution of wing shapes among theropods and early birds and their placement in two possible phylogenetic topologies. The wing symbols next to the taxon names represent the putative shape of forewings (Microraptorinae, Anchiornis, Aves), hindwings (Microraptorinae, Anchiornis, Archaeopteryx), and flight skin (Scansoriopterygidae). “X” indicates absence of wing surfaces, “?” indicates an uncertain finding. A. This topology implies multiple independent acquisition of hind wings and early single acquisition of forewings followed by multiple loss. B. This topology implies acquisition of forewings and possibly hindwings in a common ancestor of Anchiornis and the birds, and independent acquisition of both forewings and hindwings in the dromaeosaurs. The acquisition of hindwings at the Anchiornis+Aves node would imply a subsequent loss within the birds. Abbreviations: Dein, Deinonychosauria; Drom, Dromaeosauridae; Penn, Pennaraptora; Orni, Ornithothoraces; Pygo, Pygostylia; Troo, Troodontidae. Adapted from Sullivan, Xu, and O’Connor 2016.
The occurrence of a bastard wing (alula17) is also so unsystematically distributed that multiple independent origins are assumed (fig. 13). The bastard wing is important in contemporary birds for steering during slow flight (Lee et al. 2015).18 Thus, although an alula was developed in the four-winged dromaeosaurid Microraptor gui (Xu et al. 2003b), it was absent in most basal birds such as Archaeopteryx, which Zhou and Zhang (2006a, 93) find “puzzling.” The oldest evidence of an alula among Cretaceous birds is in the enantiornithines Eoalulavis (Sanz et al. 1996) and Protopteryx (Zhang and Zhou 2000). It is also possible that a convergent origin of an alula must be assumed in the other major Cretaceous bird group, the ornithurans; the oldest record was in the genus Archaeornithura (Wang, Zheng, and O’Connor 2015). However, Zheng et al. (2017, 448) suggest that an alula and associated flight capabilities were already realized at the base of Ornithothoraces (Enantiornithes and Ornithurae) and thus very early (see below). It is possible that the recently discovered Upper Jurassic genus Caihong, which is placed in the Anchiornithidae and thus in the Paraves, also possessed a type of alula (Hu, O’Connor, and Zhou 2015), but again, a convergent origin would have to be assumed in an evolutionary model.
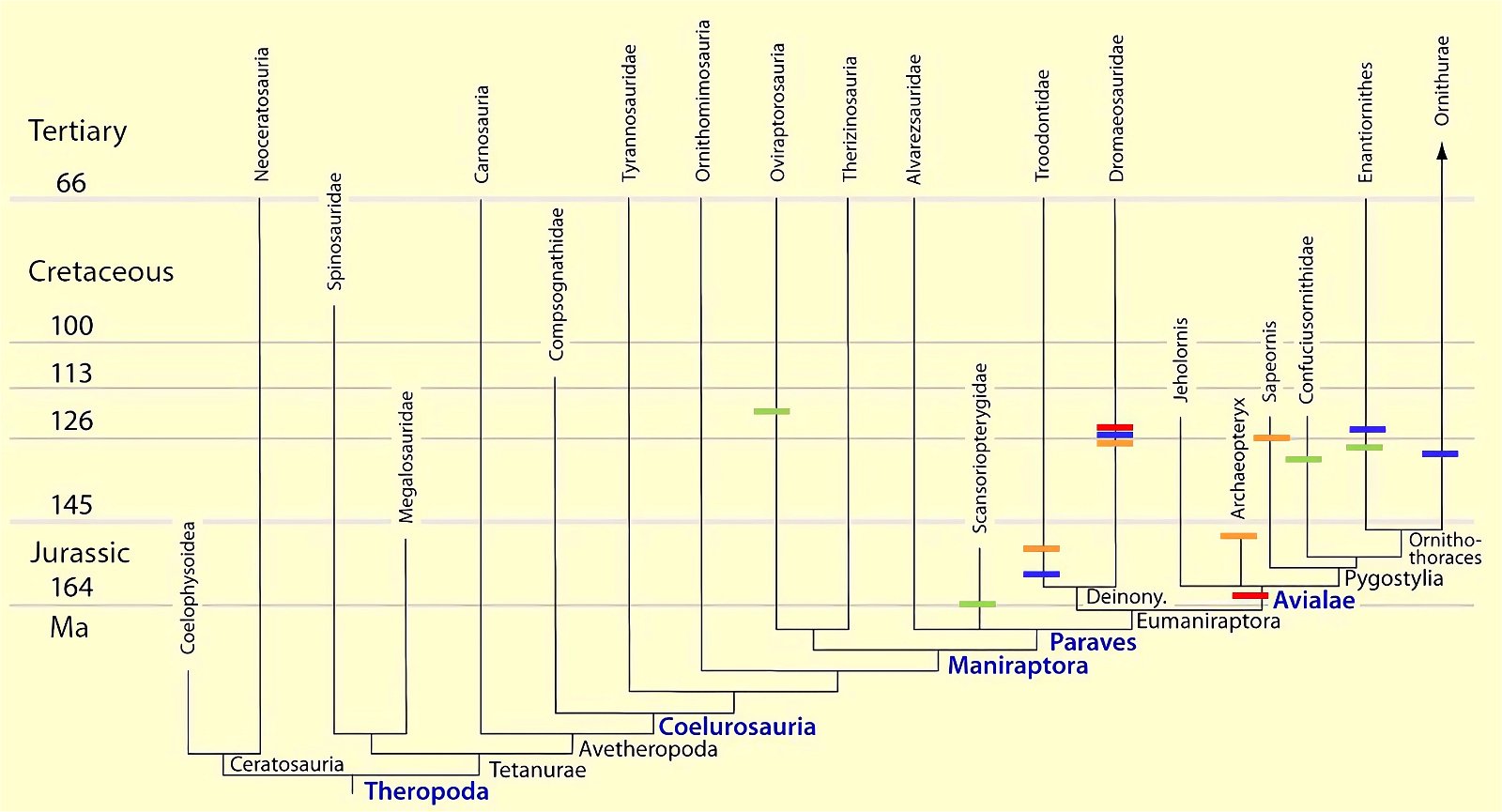
Fig. 13. Cladogram with indication of the time of occurrence with feather types that appear convergently (partly multiple). Red: wing feathers (asymmetrical): Microraptor; Anchiornis; Avialae; blue: thumb feathers: Caihong, Microraptor, Ornithothoraces (convergent twice in this group in Eoalulavis and Archaeornithura); orange: feathers on barrel: Microraptor, Sapeornis, Anchiornithidae; light green: band-like feathers: Oviraptorosauria, Scansoriopterygidae, Confuciusornithidae, Enantiornithes. (Assembled according to the sources mentioned in the text.)
Current cladograms (figs. 14, 15) illustrate the unsystematic distribution of different feather types and feather positions, and fig. 16 shows the enormous diversity of tail types that occurred relatively simultaneously in theropod genera and birds of the Lower Cretaceous and cannot be consistently classified in evolutionary sequences.
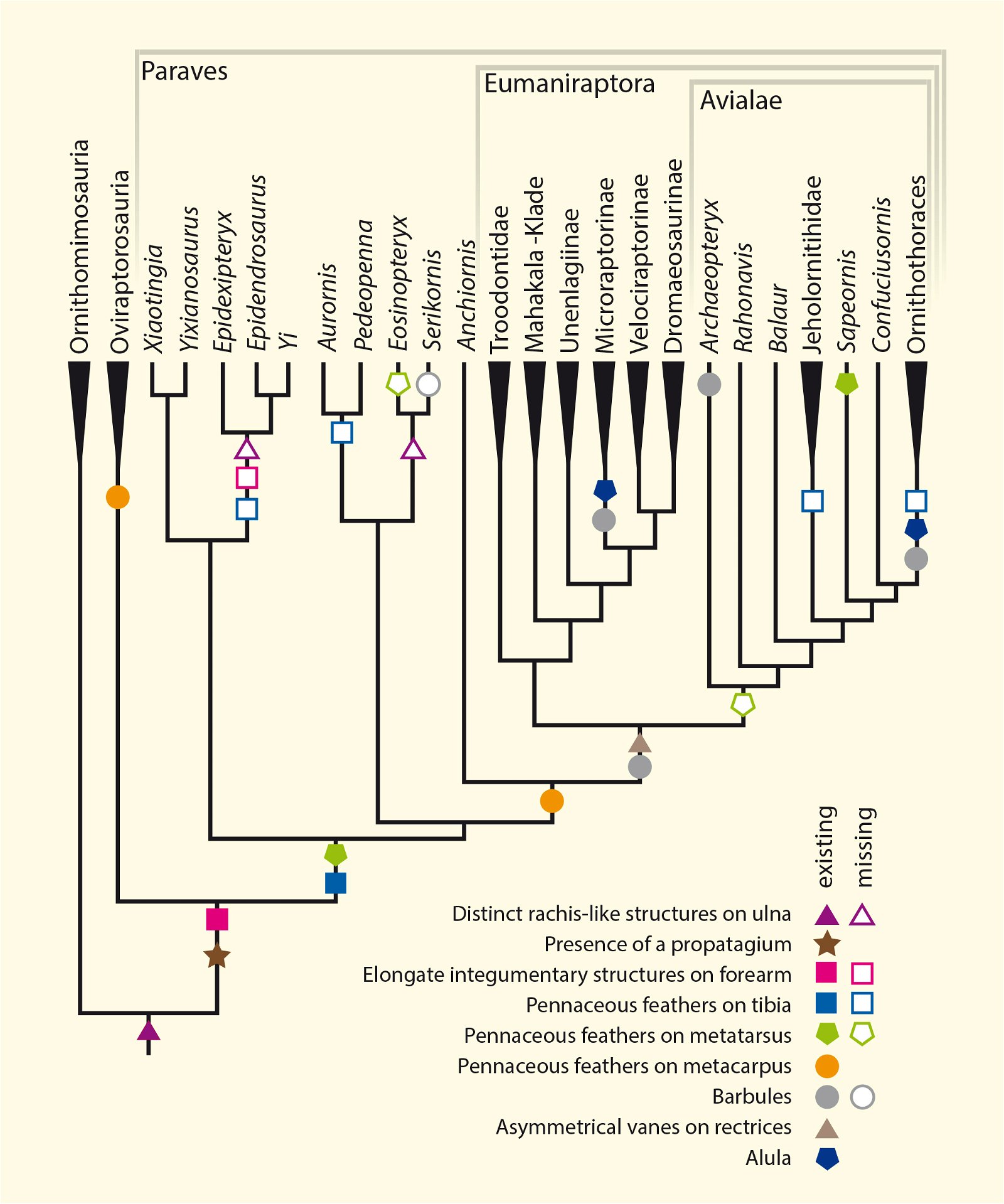
Fig. 14. Consensus tree indicating feather types based on the phylogenetic analysis of Lefèvre et al. (2017). The tree reveals numerous homoplasies.
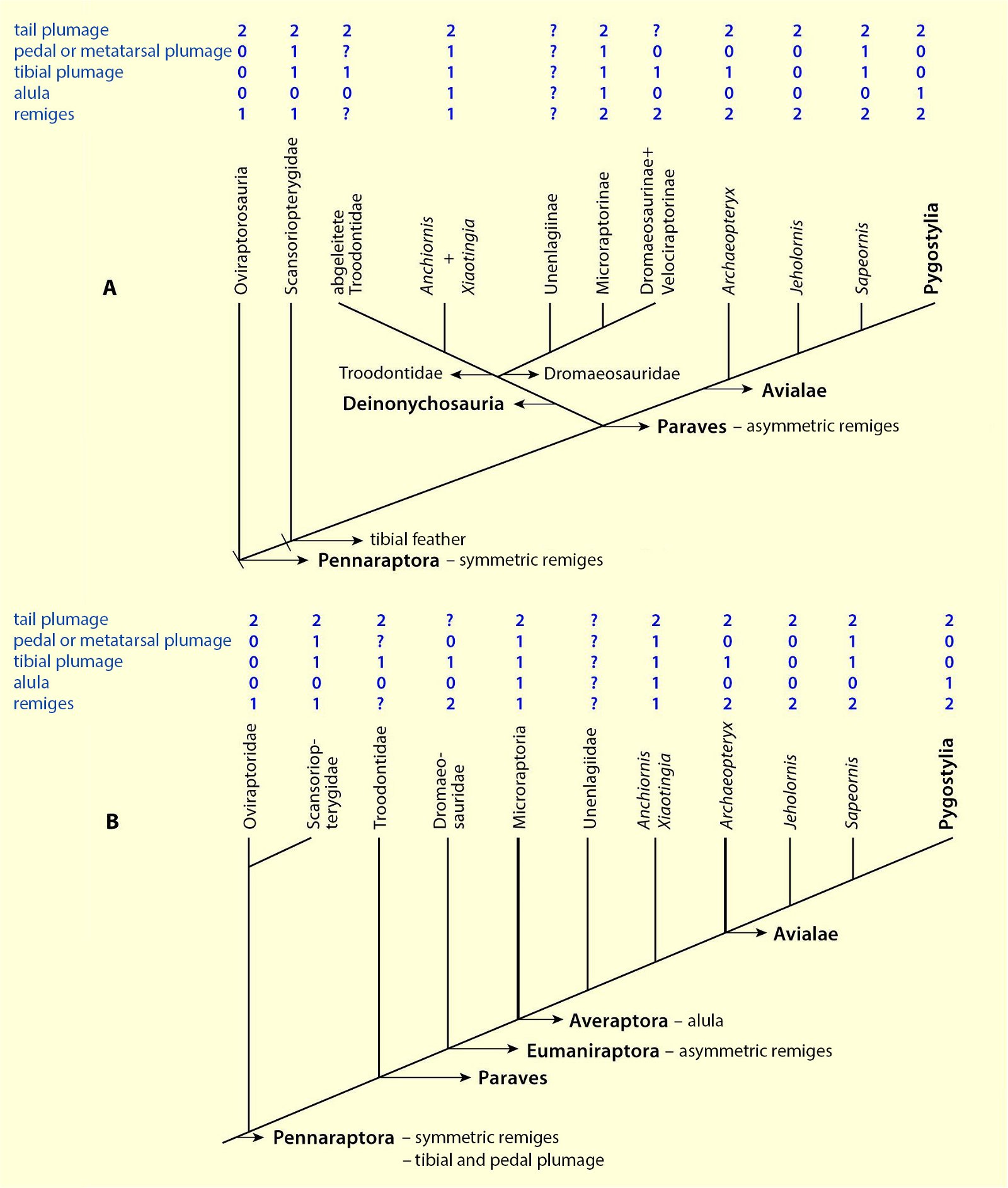
Fig. 15. Simplified cladograms of derived coelurosaurs showing which feather types are present in each group. A cladogram after Gianechini et al. (2017), B after Agnolin and Novas (2013). 0 feature absent, 1 hairy or downy appendages, 2 pennaceous feathers, ? uncertain. Particularly in variant B, there is largely no clear systematics of distributions. Agnolin et al. (2019, 21) comment as follows: “In sum, some of the diverse phylogenetic analyses put forward by different authors indicate that each feather type may have evolved convergently two or three different times, whereas other analyses indicate a single origin for flight feathers. For this reason, the origin and early evolution of the different feather types is far from being well-known and largely depends on the phylogenetic scheme adopted.” (Adapted from Agnolin et al. 2019).
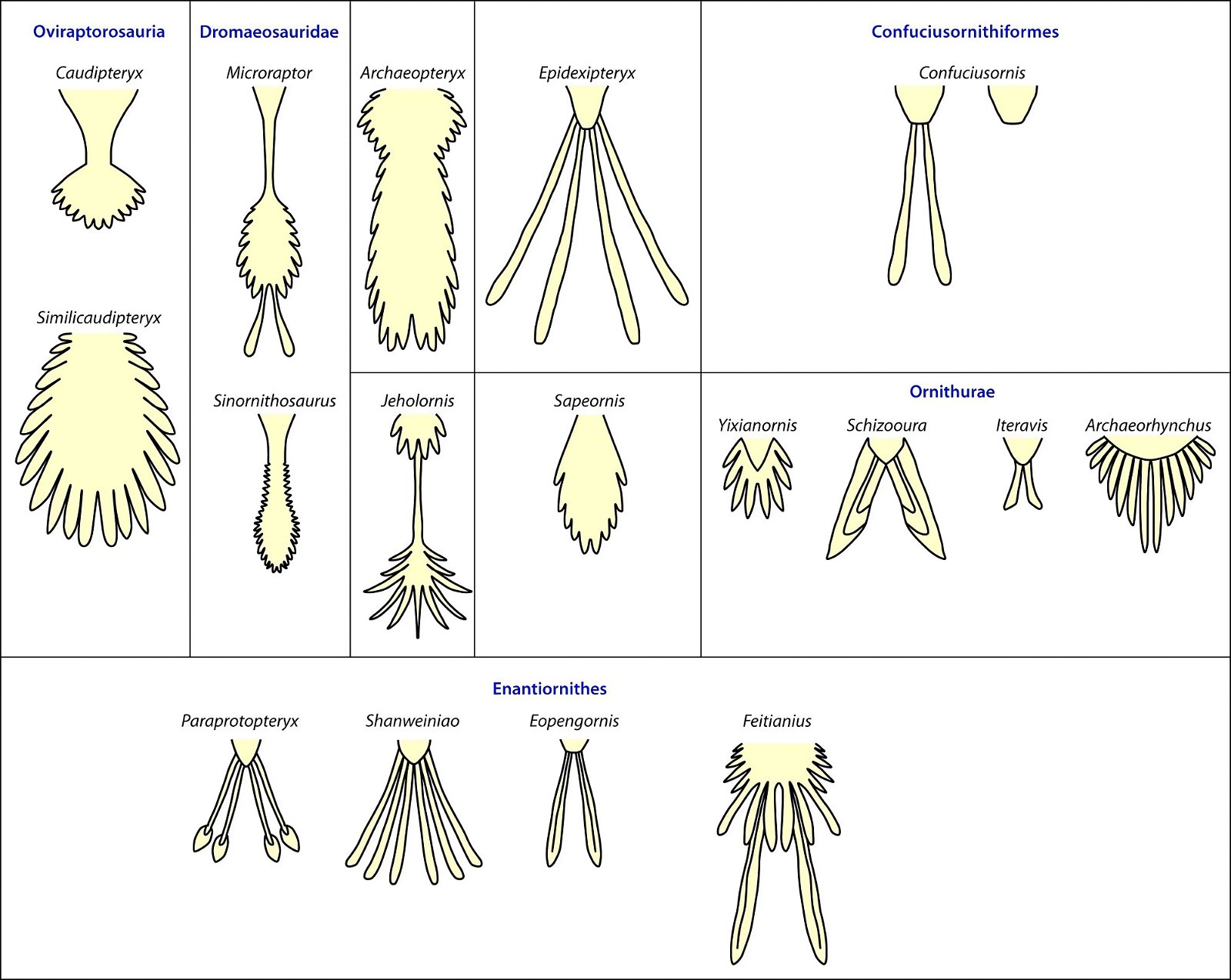
Fig. 16. Variety of different expressions of tails of birds and feathered (presumed) dinosaurs from Lower Cretaceous and Upper Jurassic (Archaeopteryx). (According to www. deviantart.com/albertonykus/art/PennaraptorTails-465558142)
Teeth and Beak
A prominent feature of birds is the bill. “Aside from feathers the bill is surely the most quintessentially bird-like feature of the avian body” (Proctor and Lynch 1993, 66). Birds use their bills not only for feeding, but also for grooming their plumage, nest building, defense, and courtship. A thin keratinous layer called the rhamphotheca covers the bony core of the upper and lower beak. The upper beak is supported by the maxilla (upper jaw) and other bones of the skull and is more or less movable by a kind of hinge joint. In most birds, the upper beak also contains two nostrils.
The bird’s beak must be seen in the larger context of bird anatomy and nutrition. Since chewing food with the beak is hardly possible, a gizzard is needed as a “substitute.” Thus, the center of gravity of food processing is shifted to a more aerodynamically favorable position at the same time (Proctor and Lynch 1993, 62).
While all modern adult birds are completely toothless,19 most fossil birds that have been recorded in Mesozoic strata possessed a toothed jaw or a combination of beak and toothed jaw (for example, Hesperornis, Martyniuk 2012, 43). The Rhamphotheca does not have dental alveoli (cavities for teeth) in any known case, even in fossil forms. In toothed birds that have a beak in addition to teeth, the beak and teeth are clearly distributed over different parts of the jaw and serve different tasks in feeding. The expression “teeth in the beak” sometimes used is incorrect (Martyniuk 2012, 43).20
The expression of teeth in dentate birds is variable, apparently depending on the type of diet (Louchart and Viriot 2011, 663). The diversity of the expressions of the teeth in dentate birds shows that they are not mere regression stages on a path to the toothless bill.
The formation of a beak is evolutionarily theorized to be related to weight savings. However, given the existence of many toothed birds on the one hand and terrestrial forms with horned beaks on the other, this relationship is not very convincing (see Wang et al. 2017a, 10930; Mayr 2017b, 72; Zhou, Sullivan, and Zhang 2019).21 There are large runners among toothless theropods for which weight saving is not an option. In many flight-capable species the possession of teeth does not seem to detract from flight ability (O’Connor 2019, 192).22 The reason for tooth reduction is therefore unclear to evolutionary workers. Natural evolutionary processes are also unable to anticipate the need to save weight.
Teeth and Beaks in Cretaceous Birds
The opposite birds common in the Cretaceous were mostly fully dentate without the beginnings of a beak, the only exception being the completely toothless genus Gobipteryx from the Upper Cretaceous of southern Mongolia, which possessed a beak (Martin and Zhou 1997). In contrast, ornithurans possessed small beaks at the anterior end of the jaw and teeth in the posterior portion (Martyniuk 2012, 43f.). Archaeorhynchus, of all species, which is at the base of the ornithurans (Wang and Zhou 2016; Zhou and Zhang 2006b, 367), has a completely toothless jaw and a flat, spoon-shaped beak.23 In evolutionary theory, toothlessness thus occurs surprisingly early. This is also true of Confuciusornis, which is considered even more “primitive” (fig. 17). The completely toothless ornithuran genus Hongshanornis also stands relatively basally and is among the oldest representatives of this group (Zhou and Zhang 2005). Finally, we should mention Jeholornis, which stands at the base of the birds and possessed only three small teeth on the lower jaw (Zhou and Zhang 2003b; fig. 17).
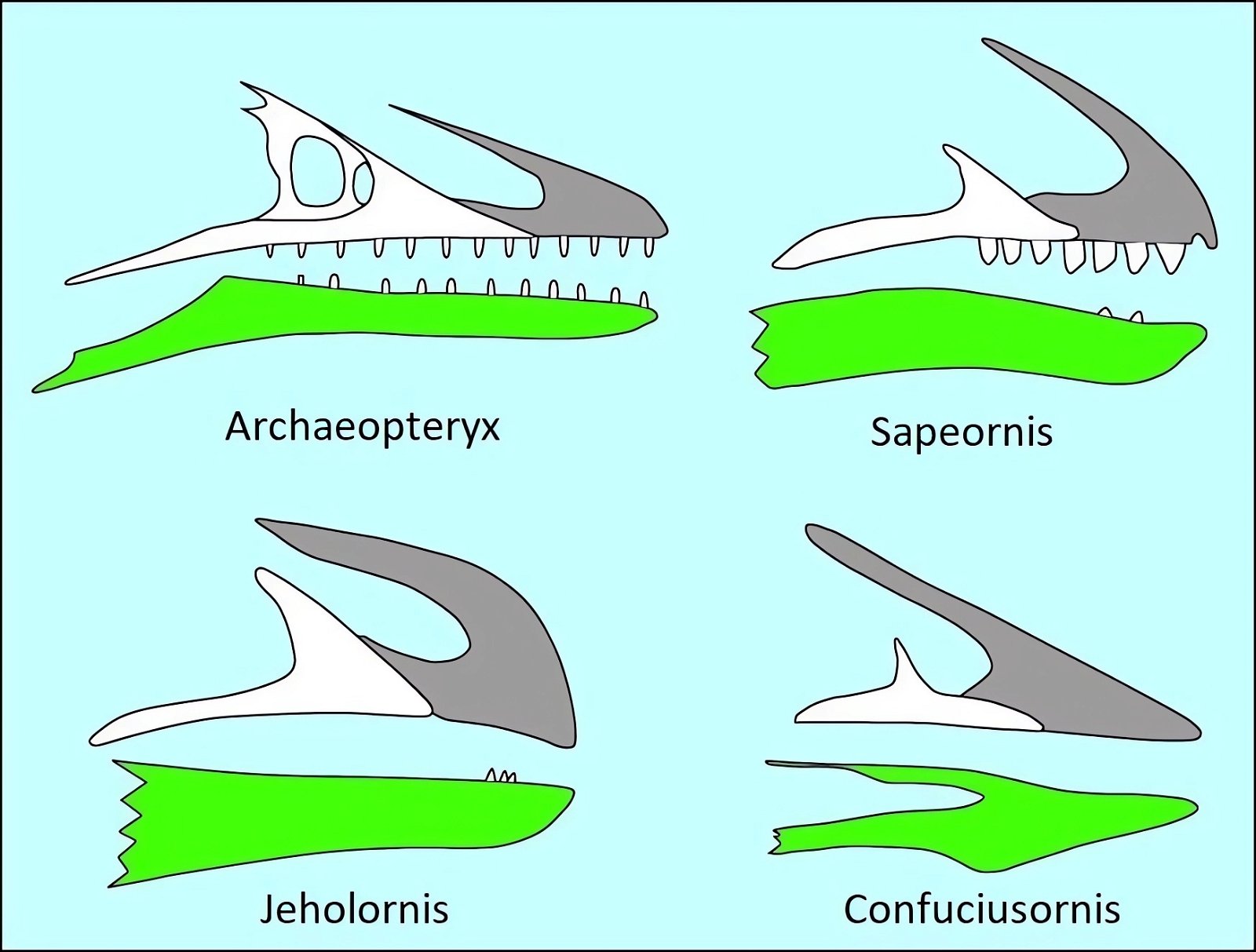
Fig. 17. Comparison of dentition in (from top left) Archaeopteryx lithographica, Sapeornis chaoyangensis, Jeholornis prima, and Confuciusornis sanctus. Premaxilla gray, maxilla white, dentals green (after Wang et al. 2017c).
Conversely, Lower Cretaceous Yanornis has more teeth than any other Mesozoic bird, suggesting that both increases and decreases in the number of teeth have occurred within Ornithuromorpha (O’Connor 2019, 192).
Tooth Reduction and Beaks in Theropods and Birds
Tooth reduction up to complete edentulism is not only found in Cretaceous bird groups, but also in a number of theropod groups. Here the Oviraptorosauria are to be mentioned first with numerous toothless genera from the Upper Cretaceous, while basal (and stratigraphically older) representatives possessed a small number of teeth (Avimimus, Caudipteryx, Protarchaeopteryx, Incisivosaurus). Incisivosaurus was distinctly heterodont (Xu et al. 2003b).
A similar situation is found in the Ornithomimosauria. Here, too, the more primitive (and older) genera possessed teeth (Pelecanimimus bore about 220 small teeth in the maxilla [at the premaxillary and maxillary] and mandible). In all other genera the upper jaw was toothless. Shenzhousaurus and Harpymimus possessed teeth in the lower jaw, while all other Ornithomimosauria were completely toothless.
Also among therizinosauria, a beak with presumed rhamphotheca was formed on the anterior part of the jaw (premaxilla) in derived genera (Erlikosaurus), with the posterior part occupied by many small teeth (Lautenschlager et al. 2013, 20657; Zanno 2010).24
Zhongornis haoae, a juvenile species of unclear systematic affiliation that is placed in the base of birds, was also toothless in lower Cretaceous rocks (O’Connor and Sullivan 2014).
Finally, Limusaurus must be mentioned. This genus of Ceratosauria possessed a fully developed beak and was completely toothless, but is placed among the basal theropods, and—unlike the other toothless forms—among the coelurosaurs (Xu et al. 2013). As with the other groups, an independent origin of the beak must be assumed (figs. 18–20).
That tooth reduction must have proceeded convergently is also emphasized in part by the fact that the pattern of tooth reduction differs among different groups. Tooth loss began in the posterior part of the jaw in basal birds and in taxa of Oviraptorosauria, whereas in Ornithuromorpha tooth loss began in the praemaxillary (Louchart and Viriot 2011; Mayr 2017a, 71).
Conclusions
Toothless forms or genera with a reduced number of teeth are so unsystematically distributed in the system of theropods and Cretaceous birds that a multiple independent origin of beaks with rhamphotheca or tooth reduction is assumed (figs. 18–20, see “quotes” below). While within individual groups (Ornithomimosauria, Oviraptorosauria, Therizinosauria, Enantiornithes) rough trends towards edentulism are recognizable, Confuciusornis, Archaeorhynchus and Zhongornis disturb this picture considerably. This is because these genera belong to the stratigraphically oldest forms with a beak and appear abruptly. Archaeorhynchus is according to phylogenetic analyses (cladogram) at the base of the Ornithuromorpha instead of at a derived position as expected by evolutionary theorists because of the formation of a beak.
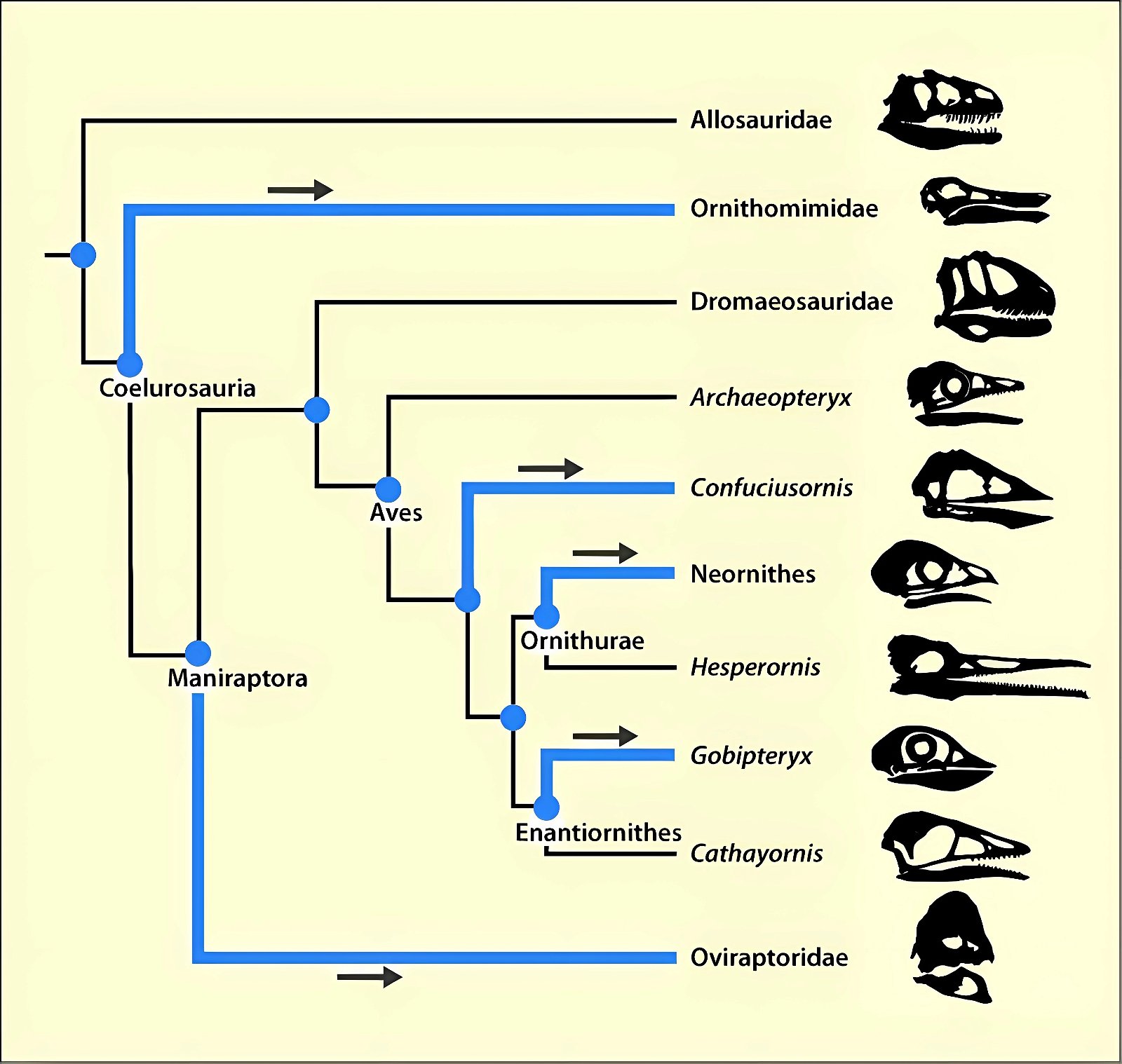
Fig. 18. Cladogram of major lineages of coelurosaur theropods showing five independent cases of tooth loss. If birds evolved, then teeth were lost independently in at least three lineages: the Confuciusornithids, the enantiornithine Gobipteryx minuta, and the Neornithes (according to Chiappe et al. 1999, 70).
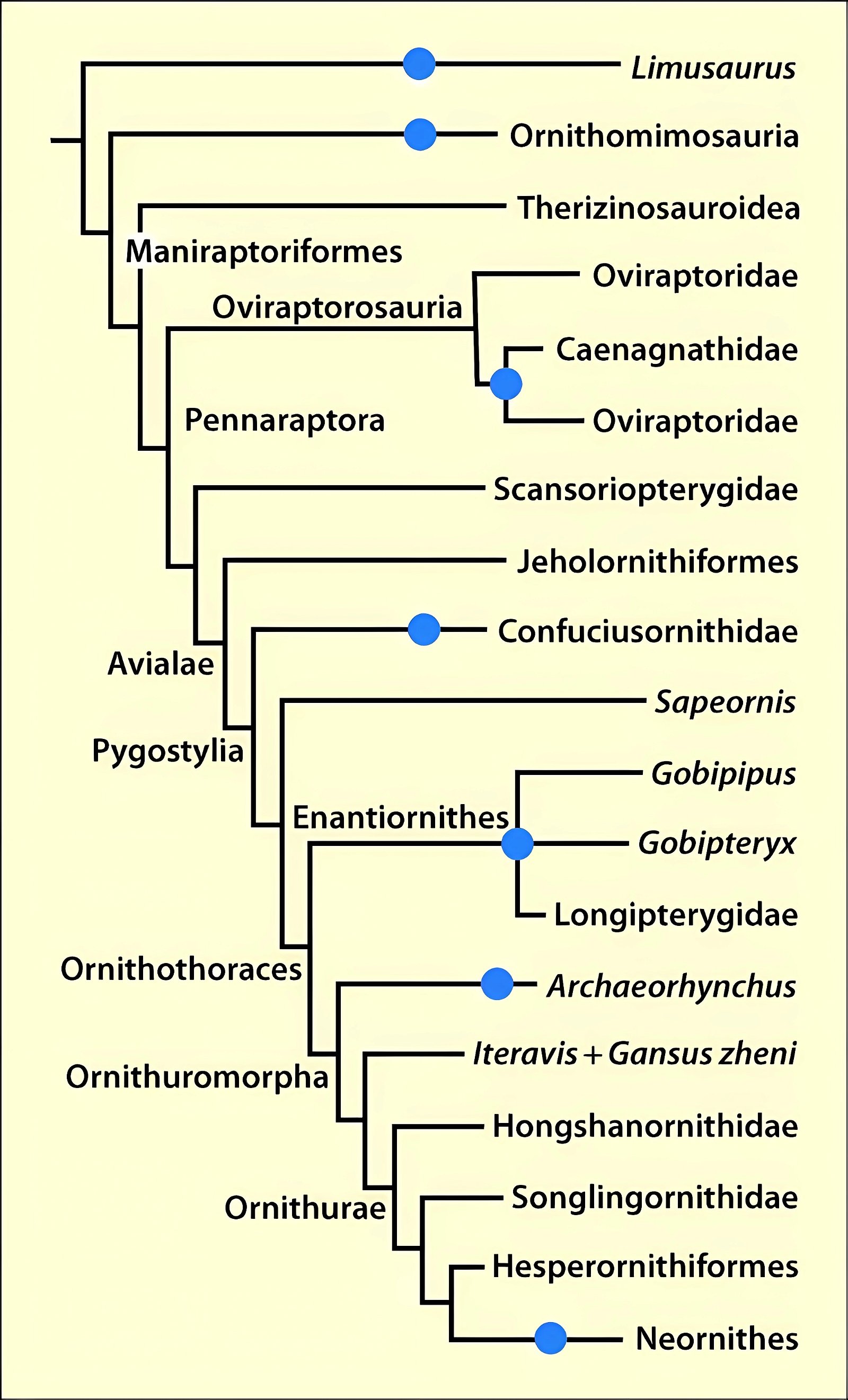
Fig. 19. At least seven independent origins of edentulism (blue dots) in theropods according to Wang et al. (2017a).
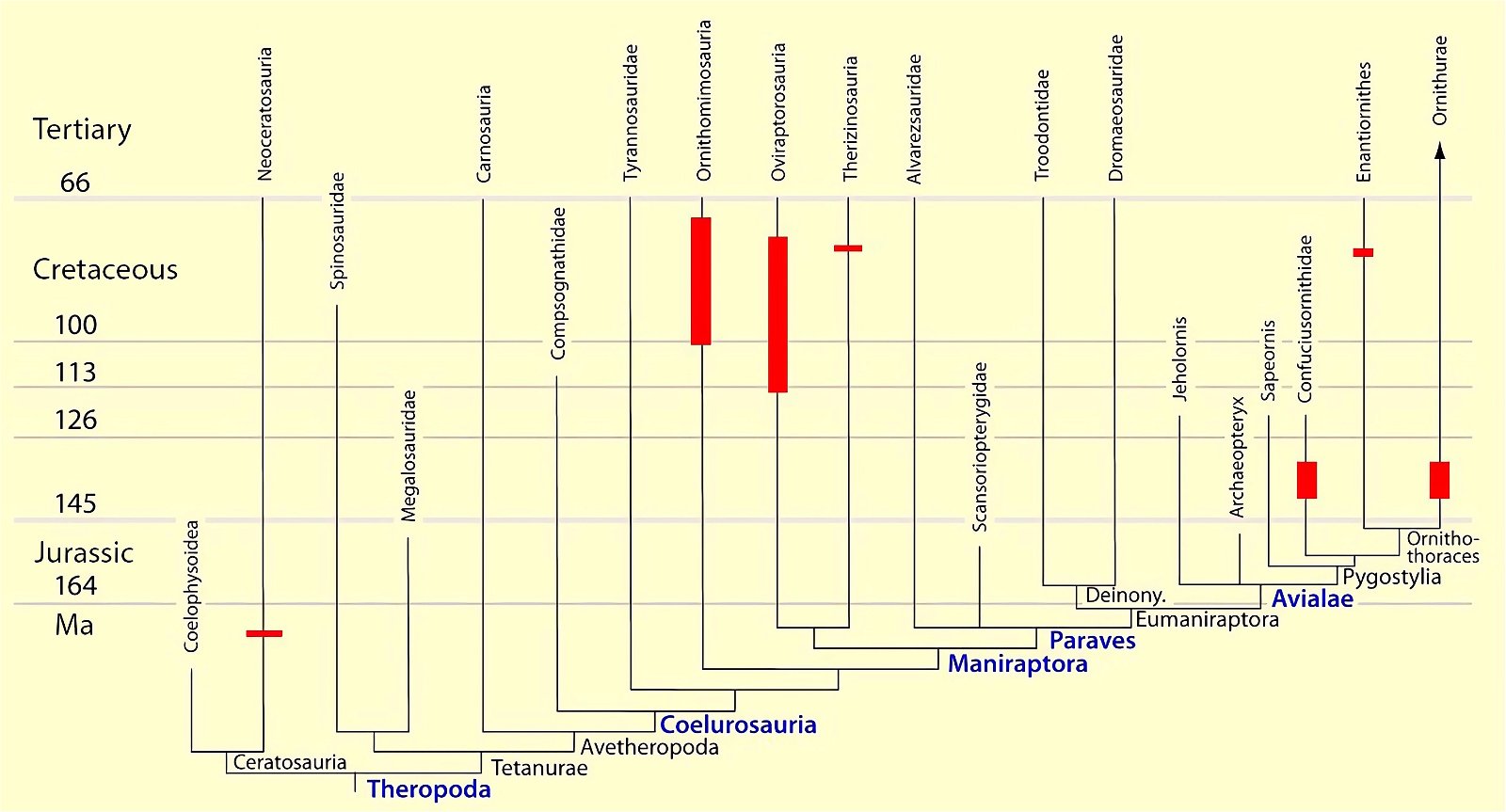
Fig. 20. Cladogram with indication of the time of occurrence showing distribution of theropod and bird groups with beaks (red markings). (Assembled according to the sources mentioned in the text.)
The distribution of toothless forms in the system contradicts with respect to the feature “beak” the thesis that bird features had already evolved in the dinosaur precursors. This is because the beaks in dinosaurs can only be interpreted evolutionarily as convergent formations that arose independently and, moreover, are usually fossilized stratigraphically much later than the oldest completely toothless bird genera (Archaeorhynchus, Confuciusornis, Hangshanornis). With Confuciusornis bird beaks suddenly appear in fully developed form and they existed contemporaneously with other forms that possessed a toothed jaw.
Quotes on Convergences in Tooth Loss and Formation of a Beak
The following citations are intended to illustrate that the fossil record requires multiple convergence of tooth loss or origination of a horned beak, but this is implausible from an evolutionary point of view.
- “The loss of teeth must have appeared several times in the evolutionary history of birds because it has occurred independently in extant birds as well as more basal birds such as Confuciusornis and Gobipteryx.” (Zhou and Zhang 2006b, 368)
- “Minimally, there are six lineages of Avialae that show evidence of tooth reduction, with four lineages exhibiting complete tooth loss. . . . Among avialaen [sic] lineages exhibiting tooth reduction or loss, a rhamphotheca has also independently evolved . . .” (Meredith et al. 2015, 1)
- “The known fossil record shows that the tooth reduction happened independently on multiple lineages of Cretaceous ornithuromorphs” (Wang and Zhou 2017, 13). Note that multiple convergence is assumed within Ornithuromorpha alone.
- “At least seven transitions to edentulism occurred independently in theropod dinosaurs, all presumably accompanied by the appearance of a horny beak.” (Wang et al. 2017a, 10930).
- “Teeth have been reduced or lost independently several times in various lineages of early avian evolution [e.g., Sapeornis, Zhongjianornis, Confuciusornithidae, Enantiornithes and Ornithurae].” (Zheng et al. 2011, 15905)
- “Tooth reduction occurred in many avian lineages and led to complete edentulism in Confuciusornithidae, the enantiornithine Gobipteryx, the basal ornithuromorphs Archaeorhynchus, Zhongjianornis, and Schizooura, as well as in Neornithes” (Mayr 2017a, 71, citing Louchart and Viriot 2011).
Brain and EQ
Birds have a greatly enlarged brain in relation to body weight compared to modern reptiles. This is especially true for the forebrain. The measure of cerebralization, which does not depend on body weight but on other factors, is also called the encephalization index.25 The ratio of the relative brain size expected based on body size to the actual relative brain size is given by the encephalization quotient (EQ).
In birds, the encephalization index is six to eleven times higher than in other animal groups; comparably high indices are otherwise known only in mammals (Balanoff et al. 2013, 93f.).26
Brain structure and a gradual increase in EQ are included amongst those avian traits that were gradually acquired already in theropod dinosaurs (Makovicky and Zanno 2011, 21).27 However, some avian brain traits and high EQ also occur independently in oviraptorosaurs (Kundrát 200728; Makovicky and Zanno 2011, 21, Balanoff, Bever, and Norell 2014, 1329). Moreover, since according to a study by Balanoff et al. (2013, 93) the relative size of the cranial cavity of Archaeopteryx was rather below average compared to some theropod dinosaurs. These authors conclude that avian EQ evolved convergently in many cases.30
Troodontids also have a higher EQ than Archaeopteryx. Their EQ is among the highest among theropod dinosaurs (Hendrickx, Hartman, and Mateus 2015; Makovicky and Norell 2004) and the high value must be considered convergent, as in oviraptorosaurs.
The brain features in basal birds and theropod dinosaurs in detail do not offer a consistent picture from an evolutionary perspective in other respects either. For example, the oviraptorid Conchoraptor has on the one hand bird-typical brain features, but on the other hand also those that are classified as more primitive compared to Archaeopteryx: “Most of the endoneurocranial attributes, however, have a less bird-like appearance in Conchoraptor than do corresponding structures in Archaeopteryx and modern birds” (Kundrát 2007, 49931; list of features on page 503). Thus, there is a feature contradiction here that forces the assumption of convergence or a secondarily flightlessness of Conchoraptor: “The data presented in this study do not allow an unambiguous assessment whether the avian-like endoneurocranial characteristics of the flightless Conchoraptor evolved convergently to those of avian theropods, or indicate a derivation of oviraptorosaurs from volant ancestors” (Kundrát 2007, 499).
Archaeopteryx is also close to present-day birds in terms of shape and features of the brain and skullcap, but less so in terms of brain size (Fabbri et al. 2017, 154632; Alonso et al. 200433; Sereno 2004, 996).
In the basal birds Jeholornis, Sapeornis, and Confuciusornis, there is no detailed knowledge about the size of the brain. Remarkable is the finding that in the Triassic archosaur Megalancosaurus the extremely enlarged skull is exceptionally bird-like (Feduccia and Wild 1993), which is most likely a convergence.
Wishbone
An important bird-typical feature is the wishbone or furcula. It is homologized with the two clavicles, which are fused together to form a V- or U-shaped or bilaterally sigmoid structure (fig. 21). In modern birds, the furcula varies considerably in size, shape, stiffness, and orientation relative to the rest of the shoulder girdle (Bock 2013, 1236; Close and Rayfield 2012, 134). Its function may also vary accordingly. The differences tend to be related to, but not exclusive of, the type of flight. U-shaped wishbones are more common in gliders, and a more curved V-shaped expression in divers (Close and Rayfield 2012, 1).
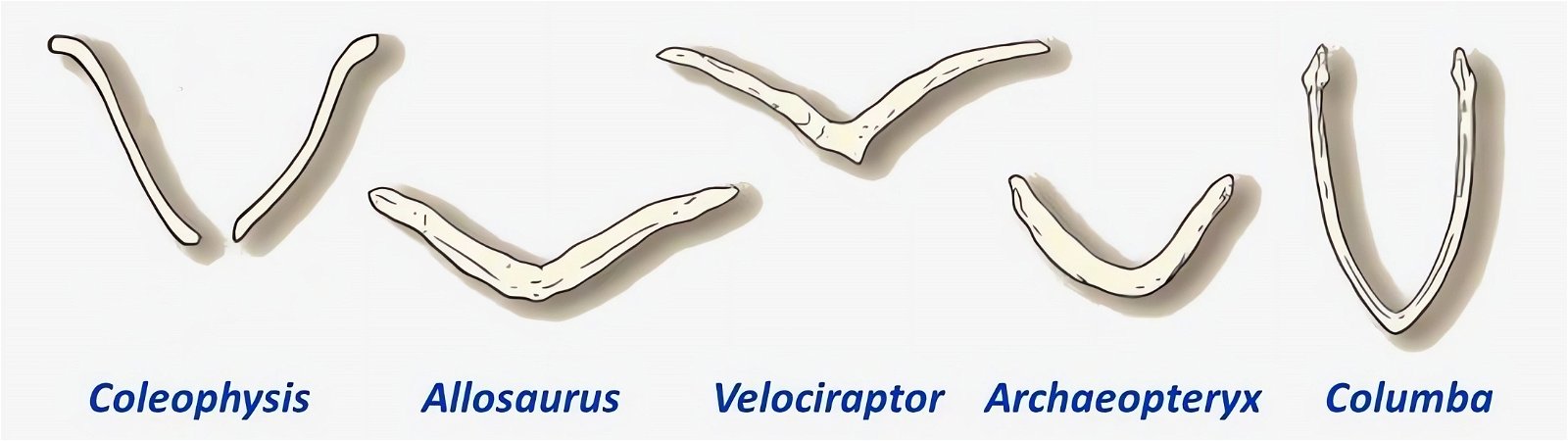
Fig. 21. Wishbones or clavicles of some theropods, in Archaeopteryx and in Columba (dove). In Coleophysis the clavicles are separated (after Padian and Chiappe 1998a).
Surprisingly, there are relatively few studies on the function of the wishbone (Nesbitt et al. 2009, 859). Frequently cited is the function as a taut spring or elastic brace between the shoulder joints that stores energy during wing flapping. The furcula also serves as a reinforcement of the thoracic skeleton to support it during the stresses of flight and as an attachment for the flight muscles, especially when flapping the wings. It also stabilizes the shoulder joint and is connected to the two shoulder blades. In addition, the furcula is thought to function in respiratory movements (Bock 2013, 1236; Jenkins, Dial, and Goslow 1988; Nesbitt et al. 2009, 85935).
Together with the coracoid bone and the scapula, it forms a special structure, the foramen triosseum (tri-bone canal, triosseal canal), a gap between these three bones through which runs a strong tendon that connects the supracoracoideus muscle (small pectoral muscle) to the humerus. This system is responsible for lifting the wing.
In fossil birds, the functionality of the furcula was probably partly different from that of modern birds. Olson and Feduccia (1979) assume that in Archaeopteryx the furcula partially replaced the weakly developed sternum as an attachment point for the flight muscles, which Bock (2013, 1238) questions. According to Makovicky and Currie (1998, 147), the function as an energy-storing feather only evolved in the Ornithothoraces.
Homology
The homology of furcula and clavicles is justified by their similar location, further by the fact that furcula and clavicles never co-occur and that both undergo similar skeletal development (Hall and Vickaryous 2015, 440).36 However, this homology is not without controversy (Bryant and Russell 1993; Feduccia 1999a, 77; Feduccia and Martin 1998; Hall and Vickaryous 2015). Feduccia considers the architecture of the shoulder of dinosaurs to be so dramatically different from that of Archaeopteryx and of modern birds that it is unlikely that any of the shoulder bones were similarly connected to the furcula and performed a similar function as in birds.37 When birds lose their ability to fly, the furcula degenerates, which is further evidence that the furcula in theropods is not homologous with that of birds (Feduccia 1999a, 77; Feduccia and Martin 1998). The occurrence of a furcula in Longisquama, a Triassic archosaur not closely related to theropods, shows that such a structure arose independently several times, so it cannot be considered a strong indication of relatedness. Moreover, the genus Velociraptor, which is discussed in connection with the question of homologisation, is 75–80 million years younger than Archaeopteryx (Feduccia and Martin 1998; see fig. 22). Norell, Makovicky and Clark (1998) express in a rebuttal the opinion that the connection of furcula and scapula in Velociraptor was as developed as in today’s birds. In addition, there are also maniraptorans with furcula already in the Upper Jurassic, a statement, which is not exactly substantiated by the authors, however.

Fig. 22. Wishbone of Velociraptor (from Norell, Makovicky, and Clark 1997; with kind permission).
Based on a review of theropods with verified furcula, Nesbitt et al. (2009, 875) conclude ten years later that there can now be no doubt that the furcula of birds is homologous with the clavicles of other tetrapods (quadrupeds). This follows from both phylogenetic and developmental data.38 However, Hall and Vickaryous (2015) later denied that homology was assured based on their studies. The furcula could also be homologous with the interclavicle. They argue that the traditional hypothesis implies a threefold loss of the clavicles in Crocodylia, Ornithischia (bird-hipped dinosaurs), and ratites, whereas if the furcula is homologous with the interclavicle, only a twofold loss should be assumed (Hall and Vickaryous 2015, 449). These two authors conclude (page 150), “We still don’t know whether the furcula represents the interclavicle, a neomorph or fused clavicles.”
Distribution
For a long time, the possession of a furcula seemed to be an avian-specific feature. In his influential standard work, Heilmann (1926) argued that birds could not be directly descended from theropods because this group had lost the clavicle and reevolution was impossible according to Dollo’s law of irreversibility of evolution39 (Hall and Vickaryous 2015, 443; Makovicky and Currie 1998, 143). However, it has since been found that the formation of a furcula was common in theropod dinosaurs (see fig. 23). Therefore, it is now thought that the furcula was coopted for flight in birds (for example, Brusatte 2017b, 54; Lipkin, Sereno and Horner 2007; Norell, Makovicky and Clark 1997).
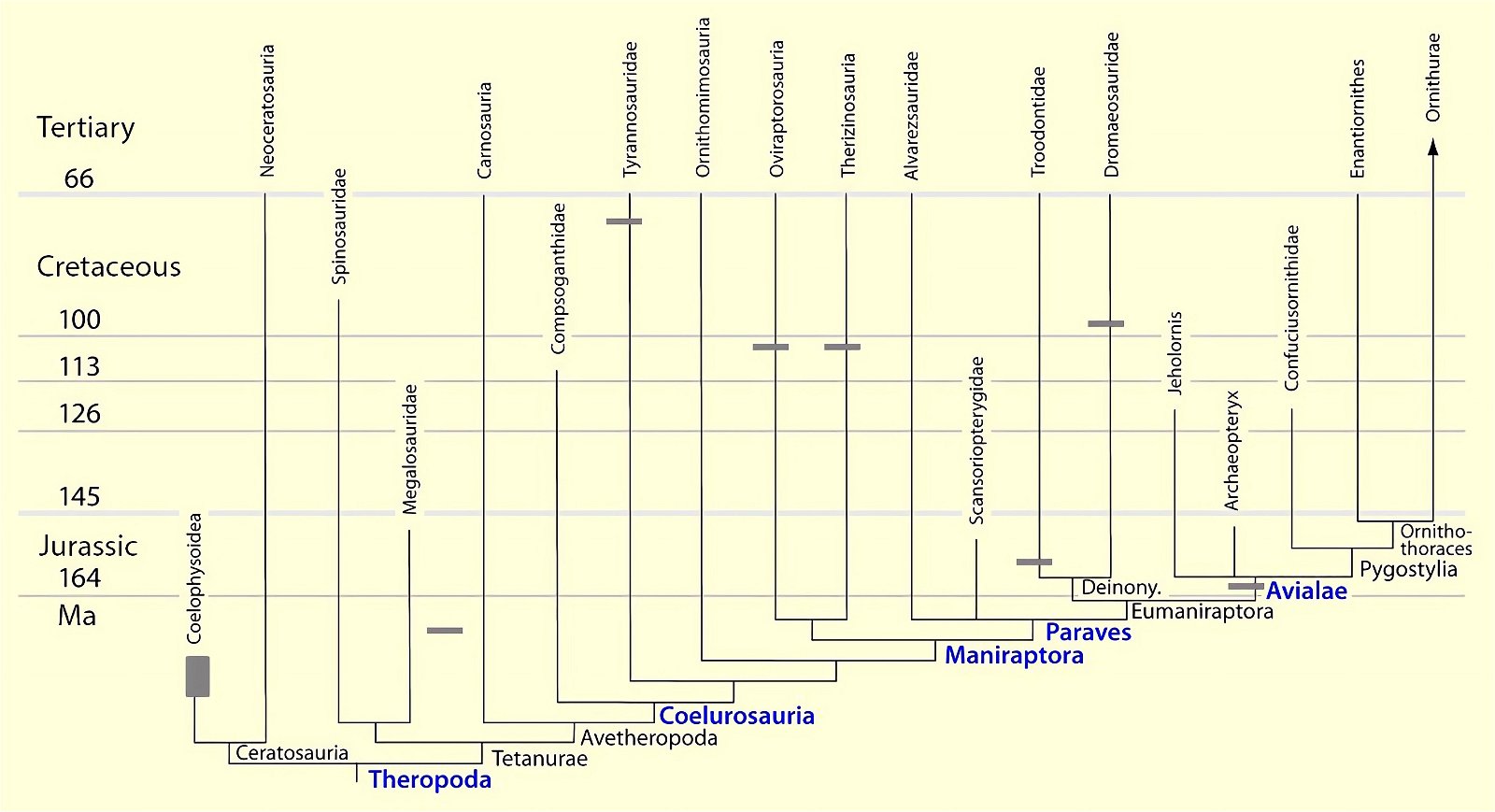
Fig. 23. Distributions of theropod and bird groups with furcula (see markings). Whether there is convergence in all cases or lack of preservation (Ornithomimosauria, Alvarezsauridae) cannot be determined with certainty at this time. The scansoriopterygids were shown to possess separate clavicles. More details in the text. (Assembled from sources cited in the text.)
The phylogenetically oldest genus in which a furcula has been demonstrated is Syntarsus in the Lower Jurassic, which belongs to the Coelophysidae, a basal group of theropods (Nesbitt et al. 2009, 872; Tykoski et al. 2002, 728).40 Nesbitt et al. (2009) provide an overview of theropod groups in which a furcula has been demonstrated and conclude that all major lineages of theropods possessed a furcula from the beginning (plesiomorphic feature). Thus, the possession of a furcula is characteristic of theropods.41 The absence of a furcula in Ornithomimosauria and Alvarezsauridae could be attributed to poor fossil preservation42 and would otherwise be most likely interpretable as a secondary loss.43 Alternatively, a multiple convergent origin of a furcula would have to be assumed, which Tykoski et al. (2002, 730f.) do not exclude. However, they point to large gaps in knowledge regarding the occurrence of a furcula in many theropod genera, which prevents a clear conclusion.44 It is still noteworthy that a furcula has not been discovered even in the scansoriopterygids, which are placed close to birds. Rather, two separate clavicles have been found in Scansoriopteryx (Czerkas n.d.; Czerkas and Yuan 2002, 6). Based on the phylogenetic position (see fig. 23), the furcula should have re-evolved into clavicles in this group—an implausible scenario.
Nesbitt et al. (2009, 873) interpret the findings of their analysis to mean that most of the features of the furcula of present-day birds evolved early, and there were only minor differences between the furcula of early theropods and more derived forms such as Archaeopteryx.45 Only the early ornithurans possessed a furcula that was typically shaped as in present-day avian lineages.46 In contrast to the relative uniformity of the shape of the furcula in fossil forms, there is great variation among present-day birds (see above).
Discussion
The furcula can be inserted only conditionally into the series of the bird-typical characteristics, which should be evolved step by step already with the theropods. If one follows the phylogenetic analyses, the furcula was already formed at the base of the theropods. Thus it is a plesiomorphic feature and as such not meaningful regarding more exact relationships between theropods and birds. Theoretically, however, within an evolutionary paradigm, the formation of a furcula can be interpreted as a pre-adaptation.
Another question is, how starting from a theropod furcula the conditions in birds evolved? The function as an elastic, energy-storing clasp requires many adjustments in the bird body. This is even more true if one considers that the furcula is polyfunctional. Changing function or integrating new functions is likely to be a formidable challenge for undirected evolutionary mechanisms. What hurdles would have had to be overcome would have to be shown by a closer comparison of forms brought into an evolutionary lineage. However, given the patchiness of the fossil record, one encounters methodological limitations here.
The homology of the furcula of different groups is obvious, but is not regarded as certain by all researchers. The fact that no furcula was found in the scansoriopterygids, ornithomimosaurs, and alvarezsaurids, despite partially good preservation, is problematic from the point of view of evolutionary theory, because it is incomprehensible that a furcula is abandoned again. This case is known for secondarily flightless birds, but one will hardly want to assume such a scenario for these three groups. Alternatively, one could assume a multiple independent emergence of a furcula. However, convergences are always problematic from an evolutionary theoretical point of view (lineages designed by means of cladism are based on the parsimony principle). Therefore, Nesbitt et al. (2009) consider it most likely that a furcula was not detected in the groups in question because of poor fossilization, that is, that its absence is a conservation artifact. In view of partly good preservation and even more in view of the formation of separate clavicles in Scansoriopteryx, this explanation is not very reasonable. However, further finds could provide clarity here.
Feduccia takes the regression of the furcula in present-day flightless birds as evidence that the furcula is a new formation in birds. He argues, “If this [loss] is a pervasive characteristic of flightless birds, why would one expect to find a fully developed furcula in flightless bipedal dinosaurs?” (Feduccia 1999a, 265). However, since a furcula occurs in these forms, it must have had a different function than in birds, which argues against its homology with the avian furcula.
Overall, despite Feduccia’s objection, there is much to be said for a homology of the furcula of birds and theropod dinosaurs, but this cannot be considered certain. On the homologizability it depends in turn whether the furcula of birds can be interpreted as a feature that was already developed in the non-flying presumed ancestors.
Gastralia, Rib Cage, Sternum
Gastralia
Features that link some theropod dinosaurs to early birds include gastralia (fig. 24). They support the thorax and serve as attachment sites for muscles in the thoracic region (O’Connor et al. 2015c, 133, 143). They may also play a role in respiration. The gastralia interlock to form a type of basket (gastral basket). They are dermal bones that are not connected to the rest of the skeleton.

Fig. 24. Left, the nearly complete gastral basket of Sapeornis chaoyangensis; middle, gastralia of Jeholornis; right, of Confuciusornis. Abbreviations: 1–16 pairs of gastralia, cor coracoid, fem femur, gas gastralia, hum humerus, pub pubis, sca scapula, stn sternum. Scale bars 10 mm each (from O’Connor et al. 2015c).
Gastralia are known from all lineages of early Mesozoic bird groups, including the basal genera Archaeopteryx, Confuciusornis, Jeholornis, and Sapeornis (for systematic position, see fig. 4). With 15–16 pairs of gastralia, the early pygostylian Sapeornis had the largest known ventral rib cage (O’Connor et al. 2015c, 135; fig. 24). Present-day birds do not possess gastralia. In them, the strongly developed sternum is thought to serve the function of the gastralia. However, there are many fossil bird species that possess both sternum and gastralia (see below). Gastralia have also been described in theropod dinosaurs and sauropods (Apatosaurus) and are found among modern lizards in tuataras and crocodilians.
O’Connor et al. (2015c) describe gastralia and their numbers in Troodontidae, Dromaeosauridae, Archaeopteryx, Jeholornis, Sapeornis, Confuciusornis, Enantiornithes, and Ornithuromorpha. Gastralia are also described in the carnosaur Aerosteon (Allosauroidea) (Sereno et al. 2008) and the oviraptorid Citipati (Tickle, Norell, and Codd 2012, 741) (both from the Upper Cretaceous; see fig. 4 for systematic position). Unfortunately, the complete gastral rib cage is known in only a few derived theropod genera, so evolutionary trends with respect to this feature can largely only be speculated (O’Connor et al. 2015c, 142).47 The size of the gastral rib cage is not particularly reduced in basal birds. The relationship between body size and the number of gastralia appears to have been markedly different in theropod dinosaurs on the one hand and Mesozoic birds on the other, leading O’Connor et al. (2015c, 144) to conclude that different evolutionary trajectories are present in the two groups.48 Their studies have also shown that, contrary to intuitive expectations, there is no correlation between the formation of a sternum and the number of gastralia in paravians and basal birds. Thus, Anchiornis (without a bony sternum) possessed about 13–14 pairs of gastralia, about as many as Microraptor gui, which possessed a sternum that was even rostrocaudally elongated.49 Jeholornis possessed only 8–9 pairs of gastralia despite being about the same size as Sapeornis with 15–16 gastralia and despite possessing a sternum quite similar to that of Confuciusornis. This situation is incongruent with the situation in other basal birds (O’Connor et al. 2015c, 145; see also Agnolin et al. 2019, 19; see figs. 25, 26).50 Only among the Ornithothoraces (Enantiornithes + Ornithuromorpha) does there appear to be a trend toward a smaller gastral rib cage with increasingly complex sternum construction.51
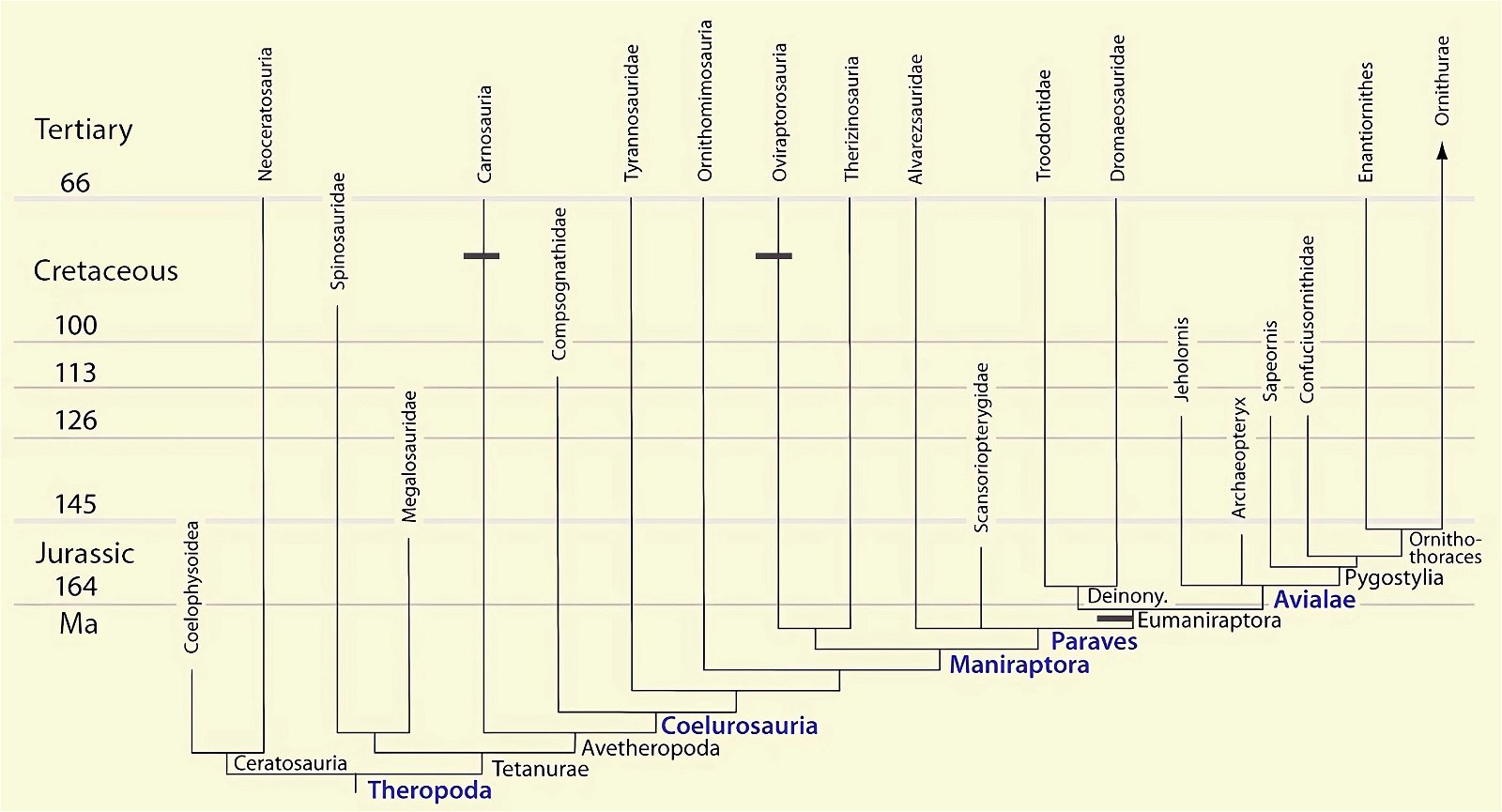
Fig. 25. Distributions of theropod and bird groups with gastral ribs (see markings). (Assembled according to the sources mentioned in the text.)
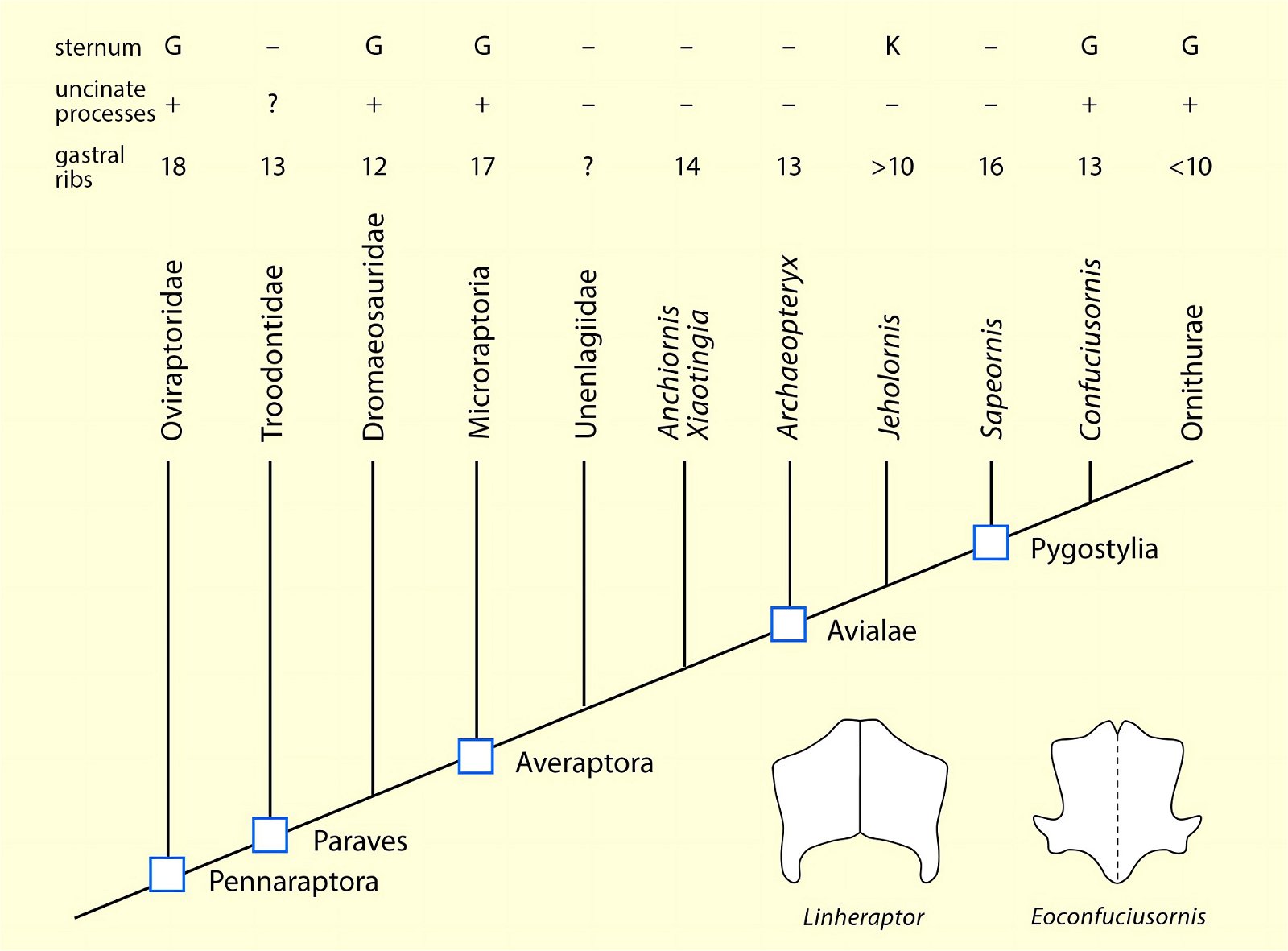
Fig. 26. Simplified cladogram of derived coelurosaurs after Agnolin and Novas (2013) showing the number of gastral rib pairs, occurrence of uncinate processes on the ribs and the relative expression of the sternum. Lower right, pectorals of the dromaeosaurid Linheraptor exquisitus and the pygostylian Eoconfuciusornis zhengi. G large, K small, + present, - absent, ? uncertain (after Agnolin et al. 2019).
The conjecture that the relatively large gastral rib cage in basal birds was related to flight and compensated for the lack of a bony sternum, while obvious, is not conclusive because, as noted, among these forms there is no clear relationship between the formation of the gastralia and the possession of a bony sternum. It is not properly understood how the musculature attached to the gastralia could have supported flight. It is possible that it had a function in respiration (O’Connor et al. 2015c, 145).
It is still remarkable that the gastralia in theropod dinosaurs are strongly derived. They cross the midline of the body and are articulated with two gastralia of the opposite side (Codd et al. 2008, 160).52 In contrast, while limited by a lack of data, there is a trend toward simplification in Cretaceous birds (see above). Thus, the conditions in theropod dinosaurs cannot be readily interpreted as precursor stages with respect to the conditions in birds. An interpretation as precursor stages is also opposed by the stratigraphic position of the theropod genera, which possess Gastralia (fig. 25).
Sternum
The sternum is where the powerful flight muscles attach for the upstroke and downstroke of the wings, and it has many functions related to flight. It is the largest bone, one of the most important and characteristic skeletal features of modern birds, and has a wide range of expression (Zheng et al. 2012, 1, 2).53
The distribution of species with an ossified sternum is puzzling from an evolutionary theoretical point of view. This is because the basal avian genera Archaeopteryx, Sapeornis, and the closely related troodontids (Anchiornis) placed in the Paraves lacked an ossified sternum, whereas one has been demonstrated in the theropod groups of Dromaeosauridae, Oviraptorosauria, and others (O’Connor and Zhou 2015; O’Connor et al. 2015c, 135, figs. 27, 28; Zheng et al. 2012). Given approximately 100 and 200 individuals studied, respectively, it can hardly be assumed that the absence is a conservation artifact (Zheng et al. 2014b).54 In evolutionary terms, this implies a zigzag course, or in other words, the distribution of this trait does not suggest a relationship with the putative phylogeny. O’Connor et al. (2015c, 135) describe this situation as confusing.55 Zheng et al. (2012, 5) consider it quite possible that the common ancestor of the Ornithothoraces (opposite birds and ornithurans, which include present-day birds) did not possess a sternum.56 In this case, however, this means that the sternum in some theropod dinosaurs cannot be included amongst the features that link the putative dinosaur ancestors of birds to birds.
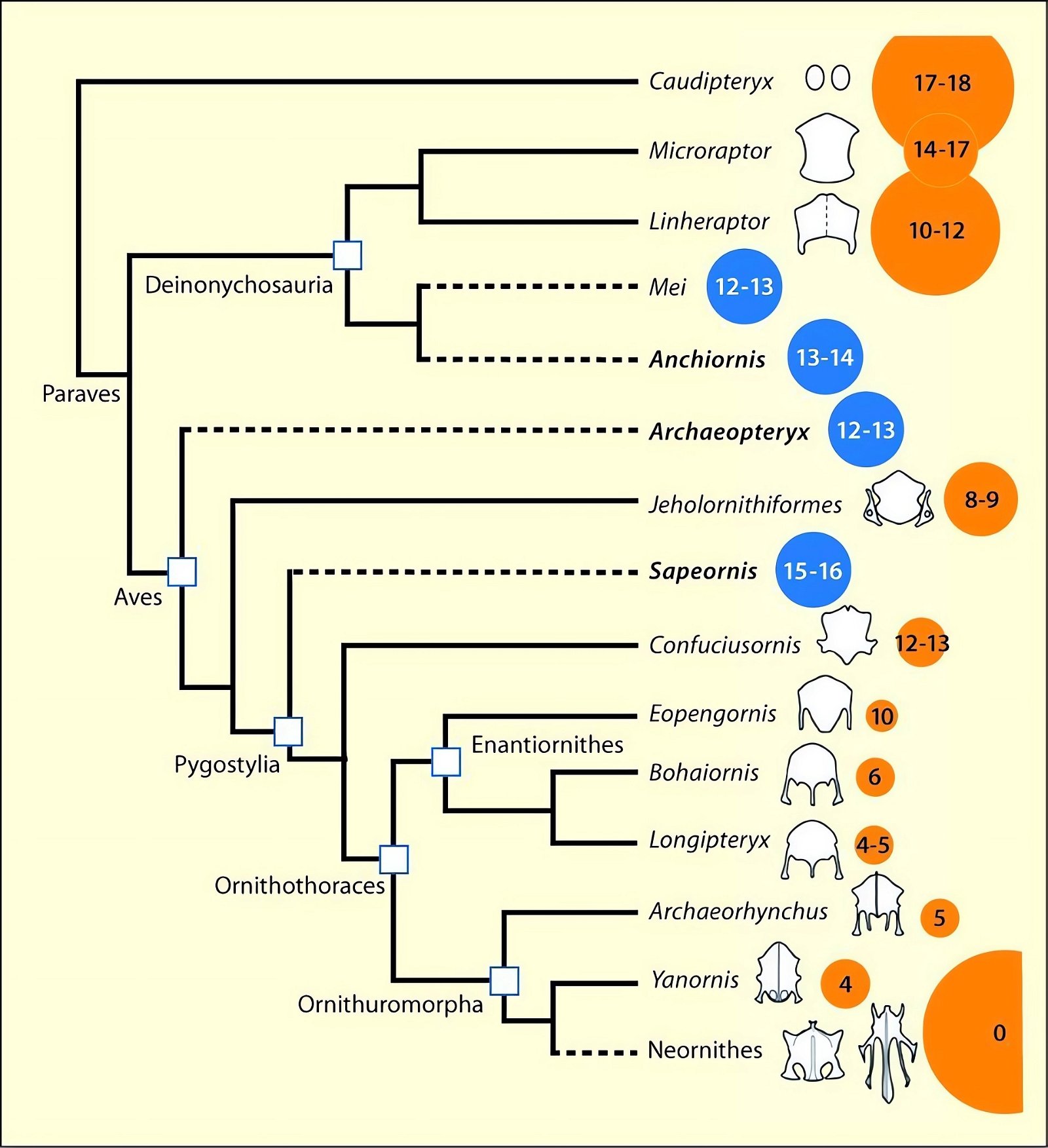
Fig. 27. Simplified cladogram of theropods showing the construction of the sternum. Size of circle reflects body size; blue and dotted lines indicate absence of sternal ossification, yellow indicates presence. The number within the circle indicates the number of gastralia; these are absent from both Neornithes (dashed line). The small light circle within the circle for the Neornithes is to indicate the extreme size difference in fossil and present-day members of this group (adapted from Zheng et al. 2014b; © 2014 National Academy of Sciences).
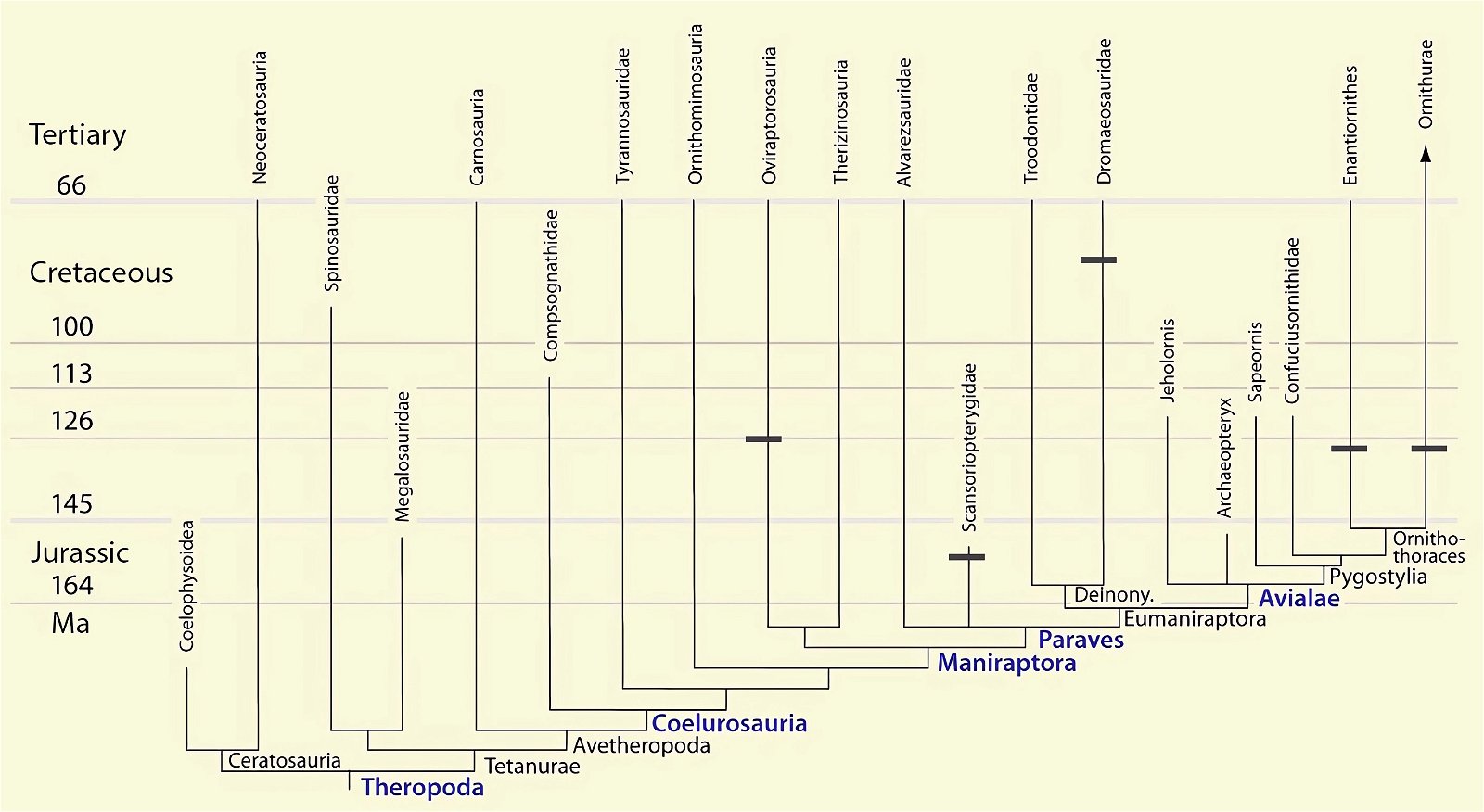
Fig. 28. Distributions of theropod and bird groups with ossified sternum (sternal plates) (see markings) (assembled from sources cited in the text).
More complex forms of the sternum with posteriorly (caudally) directed processes and a sternum keel are known only in the Ornithothoraces. One of the stratigraphically oldest genera of the opposite birds, Protopteryx, already possessed a sternum keel (Zhou and Zhang 2006a). Even among the stratigraphically oldest genera placed among the ornithurans, Ambiortus was a genus with a keeled sternum (Kurochkin 1985).57 In the basal species (which are placed outside the Ornithothoraces) the sternum is simpler and comparable to that of theropod dinosaurs. Intermediate stages are poorly known, and the development and evolution of the complex sternum are largely unknown to date, note Zheng et al. (2012, 2).58 The sternum of Mesozoic ornithurans (the lineage to which modern birds are included) is essentially modern in appearance, with various appendages, sulci (furrows), and windows, and its evolution from the simple elements of most theropod dinosaurs is unclear (Zheng et al. 2012, 3).59
In basal birds, as in dromaeosaurids and oviraptorids, the sternum (when it occurs) is formed from two medially connected plates that fuse late in ontogeny. Such a pattern is also known from present-day flightless birds (O’Connor and Zhou 2015). In contrast, in enantiornithine birds of the Jehol Group of China, the sternum is formed by proximodistally arranged median ossification centers. Therefore, this feature cannot be considered a common derived trait (synapomorphy) of the birds (O’Connor and Zhou 2015, 337f.).60 Zheng et al. (2012, 1, 2) also note such large differences in the expression and ontogenetic development of the sternum in Enantiornithes and Ornithurae that they hypothesize convergent origins despite comparable sternum complexity. Common sternum expressions should be considered parallelisms, not developmental homologies.61
Uncinate Processes on the Ribs
A bird-typical feature in the trunk skeletal region is posteriorly directed uncinate processes of the ribs. It has been demonstrated in the oldest known beaked bird, Confuciusornis, as well as in the oldest known ornithuran genus Chaoyangia (Zhou et al. 2000, 253) and one of the oldest enantiornithine genera, Longipteryx (Zhang et al. 2001, 948). Uncinate processes of the ribs also occur in dromaeosaurids and oviraptorosaurs (Zhou et al. 2000; Zhou 2004, 461; Codd et al. 2008, 157f.; Chatterjee and Templin 2012).62 Therefore, they are also included amongst the avian features that were already developed in the presumed dinosaur ancestors (Codd et al. 2008, 157). Among present-day animals, such uncinate processes are also known in the tuatara (tuatara) and developed in a cartilaginous form in crocodiles.
The uncinate processes on the ribs help strengthen the rib cage by overlapping with the following rib. They also serve as muscle attachment sites for muscles of the scapula and play a role in respiration because they are involved in its mechanics (Zhang et al. 2001, 948f.; Codd et al. 2008).63 This, in turn, is evaluated by Codd et al. (2008) as indirect evidence for bird-like respiration also in theropod dinosaurs and consequently for a very high activity of these animals.64 However, a function of the appendages in crocodilians and the tuatara as supporters of ventilation has not been demonstrated; but the muscle attachment sites in the tuatara are at least similar to those in birds (Codd 2008, 159).65 Because of these uncertainties, the function of uncinate processes in theropod dinosaurs must be assessed with caution. However, based on their research, Codd et al. (2008, 160) conclude that in these genera the uncinate processes, in concert with the specialized gastralia, sternum, and shoulder girdle, facilitated an avian-like respiratory mechanism.66
Puzzling Distribution
As mentioned earlier, uncinate processes on the ribs are known in dromaeosaurids and oviraptorosaurs in addition to birds. Codd et al. (2008) consider the uncinateprocesses of birds to be homologous with those of theropod dinosaurs and justify this on the basis of their distribution in the system (fig. 29) and the parsimony principle.67 According to Zhou et al. (2000, 253), uncinate processes in oviraptorids and in Caudipteryx (Oviraptorosauria) are barely distinguishable from those in birds. Tickle, Norell, and Codd (2012, 740) confirm that the geometry of the construction of the thorax in the theropod dinosaur Citipati (Oviraptoridae), in the basal avian species Zhongjianornis yangi and Confuciusornis sanctus, and in the ornithuran Yixianornis grabaui allowed rib movement of the same magnitude as in modern birds. These authors take this as evidence that this enabled a flow-through respiratory system in theropod dinosaurs and basal birds, and that uncinate processes of the ribs were a key adaptation for this, established before birds evolved.68
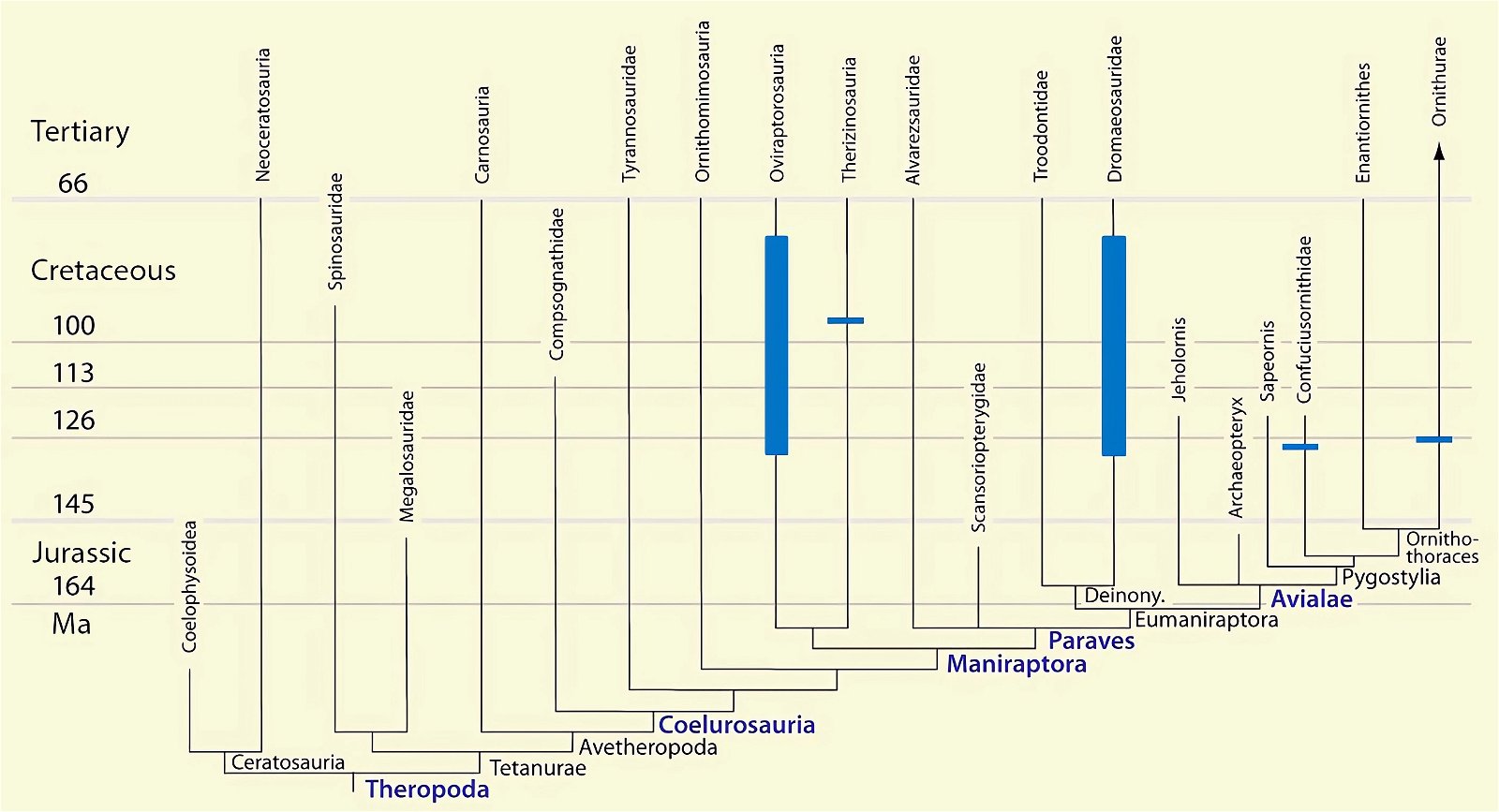
Fig. 29. Distributions of theropod and bird groups with uncinate processes on their ribs (pink markings). (Assembled according to the sources mentioned in the text)
Nevertheless, this feature is not well suited to be placed in the series of bird features in dinosaurs. This interpretation is contradicted by the fact that it is missing in the basal birds Archaeopteryx, Sapeornis, Jeholornis, and Zhongornis, as well as in the alvarezsaurids (which are close to birds) (Norell and Makovicky 1999, 27; Tickle, Norell, and Codd 2012, 744; see fig. 29). Thus, according to evolutionary theory interpretation, the uncinate processes should have been lost to reappear later (similar to the sternum, but not consistently in the same species). Alternatively, the formation is convergent despite great similarities (Norell and Makovicky 1999, 2769; Zhou et al. 2000, 25370). The possibility that the appendages were present in the above genera but are not preserved fossil (Tickle, Norell, and Codd 2012, 744) can hardly be definitively ruled out, but seems unlikely given the good preservation of the genera in question.
Paul (2001, 479) is of the opinion that features of the respiratory apparatus, such as the uncinate processes of the ribs, were more derived in dromaeosaurids and oviraptorosaurids (which are placed in the ancestry of birds) than in Archaeopteryx and were developed similarly to those of secondarily flightless birds. He evaluates this as one of the indications that these forms could have been secondarily flightless.71 But then the genera in question would not be witnesses for a connection between dinosaurs and birds with respect to the feature of uncinate processes.
Pneumatic Bones, Air Sac System and Respiration
For birds, lightweight construction of the whole body is enormously important. An air sac system (fig. 30) and pneumaticity contribute to this. Both are closely coupled with the respiratory system. The bones are air-filled by penetrating diverticula (protrusions) of the air sac system and thus connected to it. The air sac system consists of attachments to the lungs and allows for highly effective respiration (see below). Birds possess varying numbers of air sacs. Usually there are nine air sacs distributed throughout large parts of the body. They fill approximately 15% of the volume of the thorax and abdomen (Britt 1997; Proctor and Lynch 1993, 210). Air is forced into the lungs via the air sacs, as by bellows. During the breathing process, the air flows in a kind of one-way street into the posterior air sacs and from there into the lungs, further into the anterior air sacs and then into the trachea (Schmidt-Nielsen 1971; figs. 31, 32). Thus, unlike other vertebrates, there is an air circuit, which allows for a much larger respiratory volume compared to mammals, even though the lungs are very small, accounting for only 2% of the body volume.
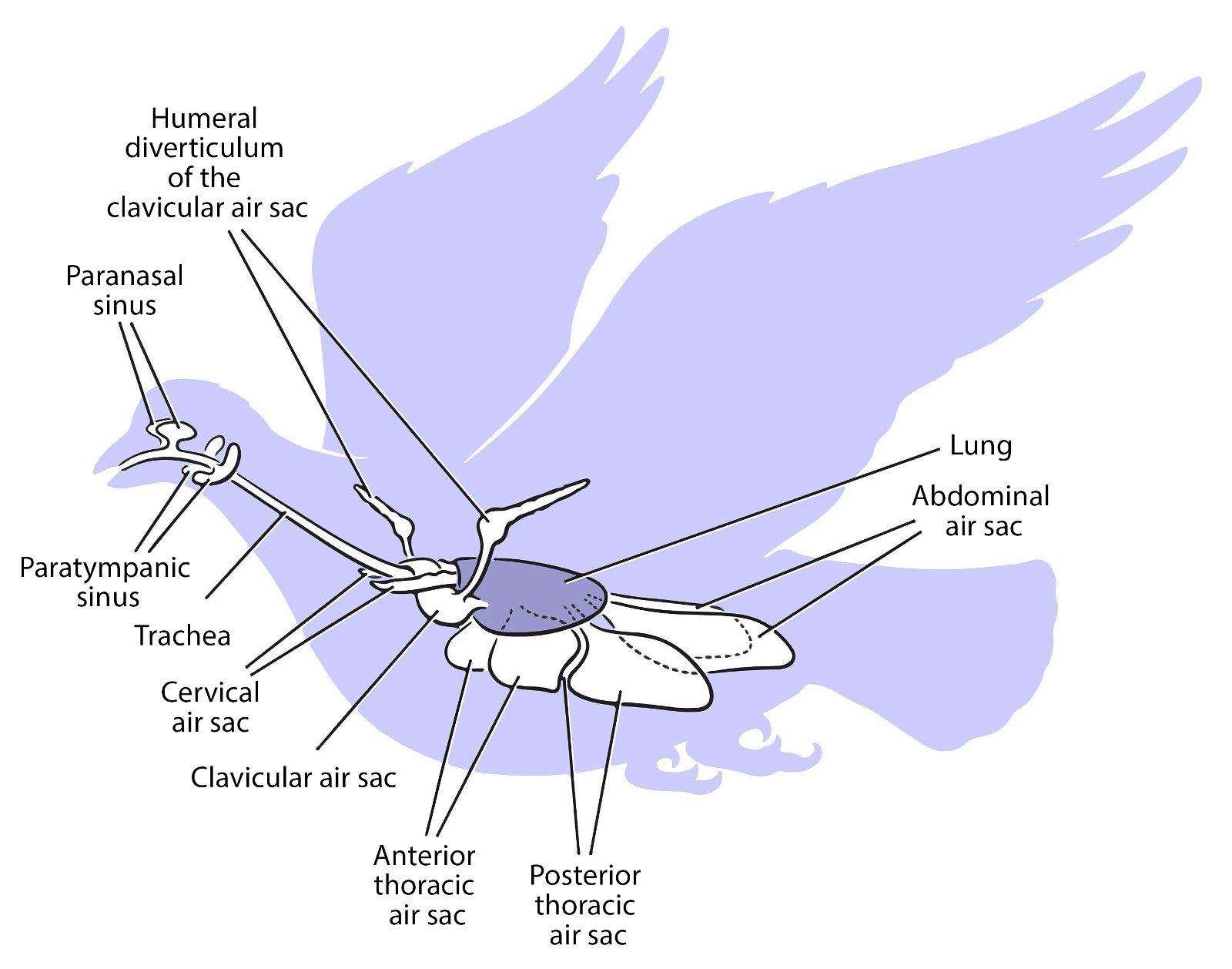
Fig. 30. Lung air sac system in birds. C. Abraczinskas, “Original caption: ‘Figure 1. Cranial sinus and postcranial air sac systems in birds. All pneumatic spaces are paired except the clavicular air sac, and the lungs are shaded. Abbreviations: aas, abdominal air sac; atas, anterior thoracic air sac; cas, cervical air sac; clas, clavicular air sac; hd, humeral diverticulum of the clavicular air sac; lu, lung; pns, paranasal sinus; ptas, posterior thoracic air sac; pts, paratympanic sinus; t, trachea,”’ https://commons.wikimedia.org/wiki/File:Cranial_sinus_and_postcranial_air_sac_systems_in_birds.jpg. CC BY-SA 2.5.
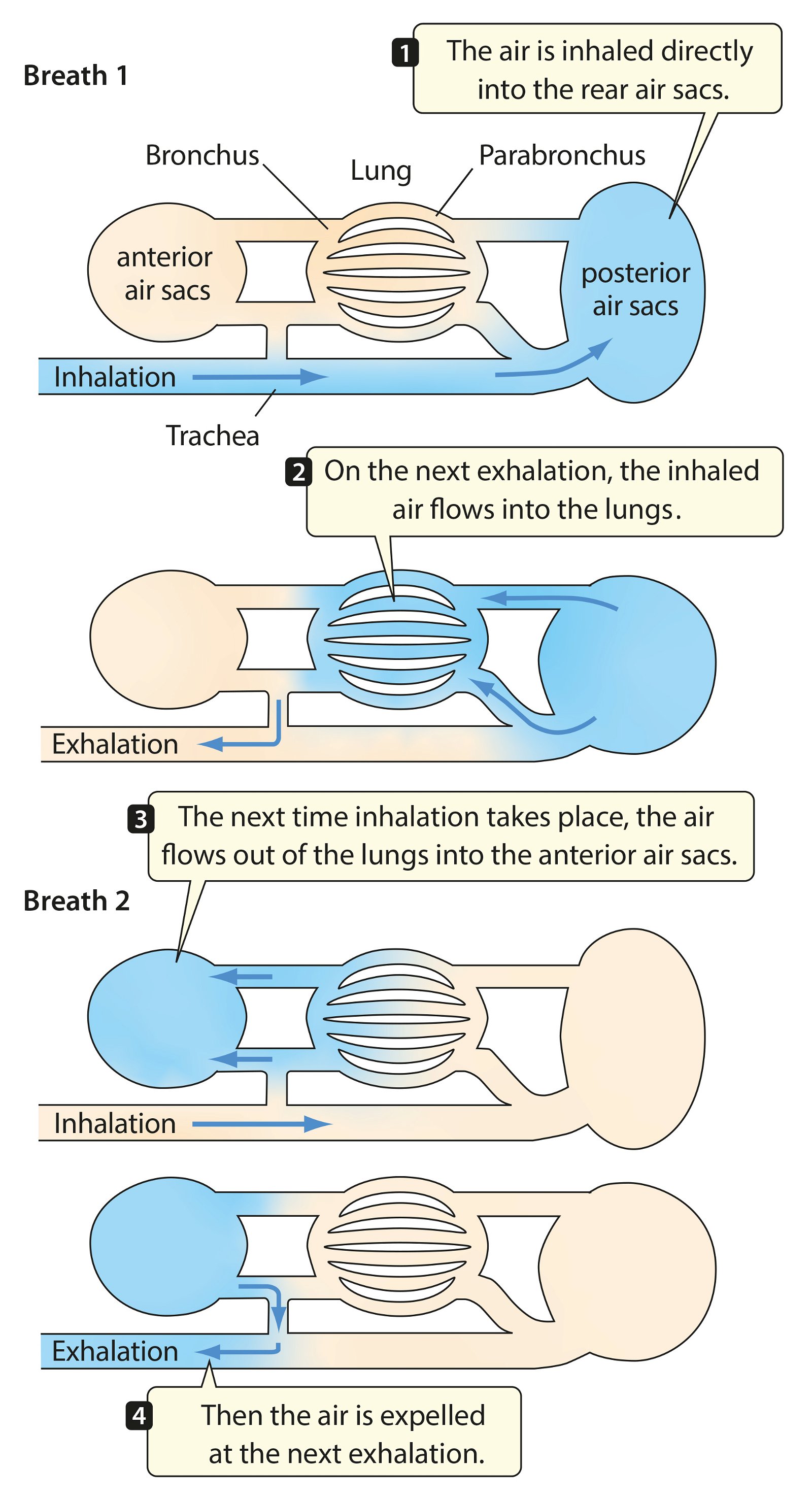
Fig. 31. Respiratory cycle in birds (after Purves et al. 2003).
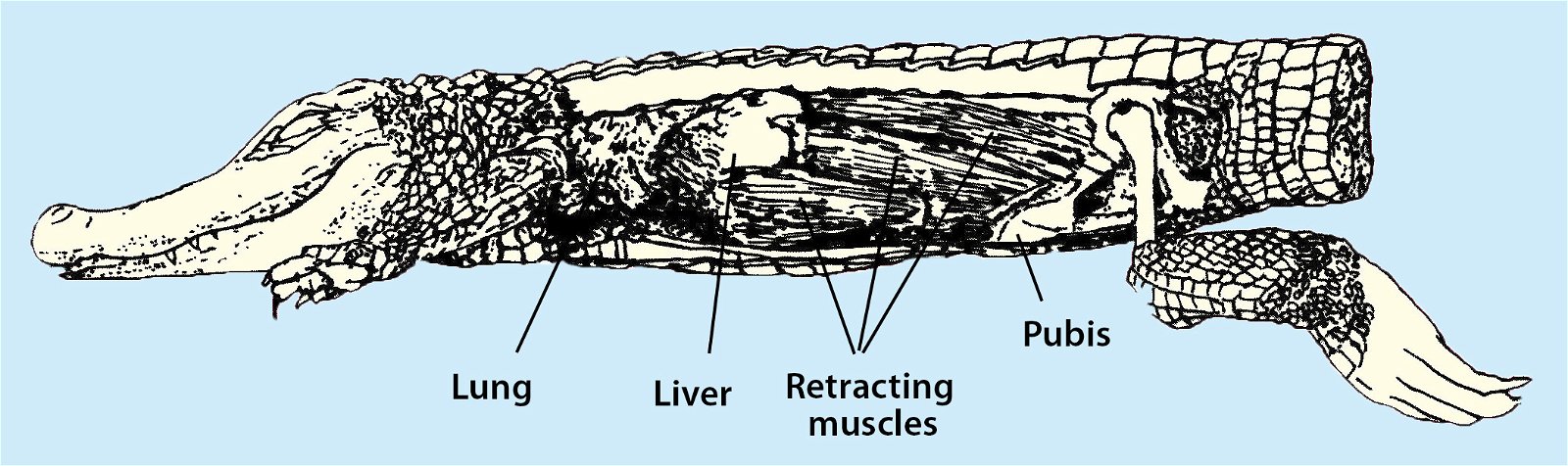
Fig. 32. Respiratory movements in crocodiles: “Recent crocodiles possess . . . a diaphragm-like membrane consisting of a thick layer of connective tissue that separates the thoracic cavity from the abdominal cavity. Although this membrane acts like a diaphragm and provides ventilation to the lungs, it does not contain muscle fibers (as is the case in mammals, for example). The respiratory movement of crocodiles comes about through a retracting muscle on the liver. The liver, with its position between the diaphragm and the retractor muscle, acts like a pumping piston. An anatomical peculiarity of crocodiles is that the liver protrudes dome-shaped into the thoracic cavity” (Zimbelmann 1999).
Schmidt-Nielsen (1971) describes the details of airflow through the air sacs and lungs. He shows that there is a distinctly sophisticated system. By leaving stale, CO2-rich air in the trachea during the breathing process, this air first flows into the posterior air sac during inhalation before fresh air is added. This is not a mistake, but is important for the consistency of the blood and the regulation of breathing: “Here we encounter one of the most elegant features of the system. If completely fresh outside air, which contains only .03% carbon dioxide, were passed through the lung, the blood would lose too much carbon dioxide, with serious consequences for the acid-base regulation of the bird‘s body. Another consequence of excessive loss of carbon dioxide arises from the fact that breathing is regulated primarily by the concentration of carbon dioxide in the blood” (Schmidt-Nielsen 1971, 76).72
It is also unusual that the lungs are constant in volume. A network of blood vessels surrounds the finest branches of the bronchial system (parabronchi) of the lung, making it a dense organ and very different from the lightly constructed lungs of other vertebrates. The diameter of the parabronchi is so small that if their inner walls were to touch, refilling would not be possible due to surface tension and the bird would suffocate. This is the reason why the lung tissue must be fixed with the help of the rib cage and by connective tissue so that the lung volume can be kept constant (Britt 1997, 591; Schachner et al. 2011, 1532).73
Breathing occurs when the ribs move apart laterally, causing the air sacs to move. This paradoxically causes air to leave the lungs when inhaling and fill them when exhaling. Unlike mammals, birds do not have a muscular diaphragm to drive respiration. The intercostal muscles are used for this purpose (Proctor and Lynch 1993, 205). A diaphragm would be obstructive because of the air sac system. Thus, air exchange is fundamentally different from respiration in other vertebrates. “Almost every part of the avian body is directly related to the respiratory system of the air sacs” (Proctor and Lynch 1993, 205).
The air sacs are also involved in vocalization. Exhalations are modulated into song in the vocal head. Finally, the air sacs and the numerous outgoing diverticula also play an important role in thermoregulation by releasing heat through evaporation. This explains why not only bones but a large part of the bird’s body is interspersed with diverticula. Thermoregulation is thus quite different from that in mammals. Sweat glands are not developed, nor would they be effective under the dense plumage. Several very different aspects of avian anatomy and physiology are thus closely coupled, on the one hand, and fundamentally different from conditions in mammals, on the other. In this regard, Proctor and Lynch (1993, 205) state that the respiratory system of birds is “surprisingly dissimilar” to that of most other terrestrial vertebrates.74
Fossil Findings
Pneumaticity of the skeleton is known among modern vertebrates only in birds, but was common in extinct archosaurs (Benson et al. 2012, 170), including all theropod lineages (O’Connor and Claessens 2005), pterosaurs, and sauropods, and mainly in the vertebrae but also in the ribs. Pneumaticity has also been detected in cervical and thoracic vertebrae of Archaeopteryx (Britt et al. 1998) and in the pelvis of the London specimen (Christiansen and Bonde 2000), which is considered to indicate the existence of air sacs (Wang and Zhou 2017, 20). Therefore, pneumatized bones are among those bird characteristics that are also present in presumed dinosaur ancestors; in the case of this feature, even common. It could be a feature that even the Ornithodira (birds, dinosaurs, and pterosaurs) have in common (Quick and Ruben 2009, 1242). Therefore, this trait is not suitable to determine more precise relationships within this group and between dinosaurs and birds.
Statements about whether air sacs were also formed in fossil preserved forms outside the birds are not possible without doubt. Their existence can only be inferred indirectly or corresponding assumptions, also about the construction of the lungs, can be substantiated by indirect evidence.75 Pneumatized bones are also important for weight saving. Schachner et al. (2011, 1533) point out that pneumatization of bones has no function in respiration and many findings indicate that it is primarily for weight saving, so pneumaticity does not necessarily indicate the existence of air sacs.76 However, the evidence of pneumatized bones with air pores is strong evidence for the existence of air sacs, according to Britt (1997, 591).77
O’Connor and Claessens (2005) found pneumatic openings in cervical, thoracic, and pelvic vertebrae in a fossil specimen of the primitive Upper Cretaceous theropod genus Majungatholus (later renamed Majungasaurus). They conclude on the existence of corresponding air sacs, whose diverticula are to be assigned to different vertebral bodies. The lung construction plan of the birds with flow of the air had been developed with it already with a basal neotheropodan, which is not more near related with the birds.78 Thus, it could be assumed that flow-through breathing was probably a general characteristic of theropods (O’Connor and Claessens 2005, 253) and not a new acquisition specifically related to the ability to fly.79
This conclusion is supported by a study by Sereno et al. (2008) on the also “original” allosauroid genus Aerosteon (like Majungasaurus from the Upper Cretaceous). The fossil in question exhibits extreme pneumatization of bones, including the furcula, ilium, and gastralia. The researchers conclude that diverticula of the air sac system were formed in the thorax tissues.
Wang and Zhou (2017, 20) also argue that the pneumatization of bones in several theropod lineages indicates that the “modern” avian respiratory system evolved prior to the origin of birds.80
Bird-like respiration could also be indicated by the presence of uncinate processes on the ribs, which are also found in some theropod dinosaurs (for example, oviraptorids and dromaeosaurids such as Velociraptor, Deinonychosaur, Microraptor [Codd et al. 2008]). This is because these are involved in the mechanics of respiration, but this relationship is not compelling (Zhang et al. 2001, 948f.; Codd et al. 2008, 157, 159; Tickle, Norell, and Codd 2012; see above for more details).
Controversy About the Respiratory System of Theropods
Quick and Ruben (2009) consider that some anatomical features of many theropod dinosaurs, such as the small sternum and lack of a bird-like thorax, the three-pronged pelvis, and the construction of the legs, contradict the possibility of bird-like respiratory movements because all of these are associated with breathing. The femur of birds is relatively firmly seated, and bone and musculature of the femoral region, along with the synsacrum and posteriorly oriented pubis, are needed to support the body wall, which must be understood in the context of the air sac system and helps keep the avian lungs from collapsing. In contrast, the femurs of the dinosaurs were mobile and therefore could not have fulfilled this task (Quick and Ruben 2009).81
In addition, there are (however controversial) indications that the theropods Sinosauropteryx and Scipionyx from the group of coelurosaurs had a respiratory system similar to that of crocodiles. Indeed, Ruben et al. (1997, 1999) found darkly colored protruding areas in the abdominal region of these two fossil genera that could be traces of the former liver, which in reptiles plays a special role in respiration as a kind of piston by allowing the lungs to expand and contract again by means of muscles and a diaphragm-like septum. In addition, the posterior section of the abdominal cavity appears to have been distinctly separated from an anterior heart-lung section. In Scipionyx, scientists also believe they can discern muscle fibers running from the pelvis to the presumed liver, a situation similar to that in present-day crocodilians (see fig. 32). Overall, these findings suggest reptilian respiration that is distinctly different from the flow-through respiration of birds. Meanwhile, simple flow-through respiration has been demonstrated in crocodilians and alligators, but it functions without an air sac system and much differently than in birds (see below).
Some paleontologists, however, dispute that the fossil evidence allows the interpretation of Ruben et al. 1997; 1999). For this the fossils are too strongly flattened and the fossil evidence is not clear (Paul 2001). Wellnhofer (1999) also points out that even if Ruben et al. (1997, 1999) interpret the fossil findings correctly, this is not a counterargument against an evolutionary transition from dinosaurs to birds, because a transition from a reptile lung to a bird lung could have occurred at a later stage of evolution.
In addition, Paul (2001, 479) believes that there was a gradual acquisition of bird-like features of the respiratory system in theropods and that the thorax of the most bird-like dinosaurs was essentially bird-like in construction. However, some features of the respiratory system, such as ossified uncinate processes of the ribs, sternal ribs, the presence of up to five sternal rib joints, and long sternal plates, were more derived in dromaeosaurs, Caudipteryx, and oviraptorosaurs than in Archaeopteryx. Remarkably, these adaptations resembled those of secondarily flightless birds, which could be taken as an indication that bird-like dinosaurs could also be secondarily flightless (see below).
As mentioned above, there are correlations between the special properties of the avian lung (volume constancy, very sensitive parabronchia) and features of the skeleton (especially ribs and vertebrae) that ensure that the lung volume remains almost constant. Schachner et al. (2011) examined the axial skeleton of a number of very different extinct archosaurs for skeletal features related to lung construction. They found no evidence on the vertebrae of a liver-mediated pump-piston mechanism in any of the taxa studied, but found features that argued for the existence of a rigid lung.
When or in which group of forms the bird-like respiratory mechanism first developed under evolutionary theoretical conditions is ultimately difficult to clarify for methodological reasons, since there are too many unknowns and the coupling of skeletal features with features of the respiratory system is fraught with uncertainties. In any case, a reorganization into avian lungs would require considerable reorganizations because of the abovementioned interconnections, regardless of when and in which lineage this occurred. Realistic evolutionary theoretical modeling would have to take this into account. Ruben et al. (1997) consider an evolutionary transition problematic because it would be stopped by selection at an early stage, and because a bipartite body cavity, as crocodiles possess and need for respiration (see above), would prevent the creation of air sacs. For this would require the abdominal cavity to be separated from the thoracic cavity, which would require a rupture of the diaphragm and destroy its function before further “remodeling” could even begin.82 The reorganization of thermoregulation would face similar problems. These significant evolutionary problems arise regardless of when or in what lineage the respiratory system of birds evolved.
Decision by a New Study?
In a large-scale study, Brocklehurst, Schachner, and Sellers (2018) recently showed that theropod dinosaurs, as well as other dinosaur genera, have features of the vertebrae, ribs, and costovertebral joints83 that are also typical of birds and, in them, ensure that the lungs are held immobile. The inside of the chest wall was furrowed, which is important for the fixation of the lung. The immobility of the lungs, in turn, is one of the prerequisites for bird-typical through-flow breathing. This extensive study seems to have finally clarified that dinosaurs possessed a highly efficient respiratory system similar to that of birds. In evolutionary theory terms, this means two things:
- The effective avian lung is one of the features that was realized before birds evolved, and
- The origin of the bird lung is not connected with the origin of the bird flight.
However, the bone structure of the vertebrae and ribs is not proof of a bird-like lung.
How respiration with immobile lungs evolved is not answered by the research of Brocklehurst, Schachner, and Sellers (2018) (nor was that the goal of the study). Rather, indirect osteological evidence showed that no evolutionary stages to this particular lung structure are detectable among the Dinosauriformes (Brocklehurst, Schachner, and Sellers 2018, 9).84
Direct evidence of fossil preserved lungs is believed to have been provided by Wang et al. (2018) in the Lower Cretaceous basal ornithuromorph Archaeorhynchus. The lungs are reported to be very similar to those of living birds. This is remarkable in two respects:
- Archaeorhynchus is placed at the base of the ornithuromorphs; thus a “modern” bird lung, from an evolutionary theoretical point of view, was established early.
- The skeletal features corresponding to the construction of the lung are “primitive”85; a correlation skeleton—lung is given only with restriction, which means in the reverse conclusion that from the construction of thorax and vertebral column the construction of lung and respiratory system cannot be concluded with certainty.
Pneumatization of the Bones and Breathing
Inferring an avian respiratory system from the pneumatization of bones seems rash, as pneumaticity may have had other functions, particularly weight saving, for which there are again reasons other than flight capability. This is evident from an extensive comparative study that examined 158 theropod dinosaur taxa, 131 of which had pneumatized bones (Benson et al. 2012). The authors conclude that an increase in pneumatization of bones occurred independently in 12 lineages, a “remarkably high number of independent acquisitions of an avian-like trait.” They say it is striking that lineages in which there are also genera with large body sizes are most affected. This correlation, however, is less striking in lineages more closely related to birds, he said. Since pneumaticity is so widespread, adaptation to flight is out of the question as an explanation; more likely is energy conservation in view of increasing metabolic performance, also in connection with endothermy.86
However, the Benson et al. data confirm, in the researchers’ estimation, that the patterns of occurrence of pneumaticity in theropod dinosaurs are similar to those in birds and were already developed in basal theropods (Benson et al. 2012, 186).87 An increase in the maximum extent of pneumatization of vertebrae had occurred abruptly in several lineages in the Upper Jurassic, at the same time that this had also occurred in many lineages of pterodactyloid pterosaurs and sauropodomorphs (Benson et al. 2012, 187).88
Flow-Through Respiration in Contemporary Reptiles
An air circuit during respiration has now also been demonstrated in reptiles living today, but without an air sac system and without pneumatized bones. Farmer (2010) and Farmer and Sanders (2010) report this in the alligator and Schachner, Hutchinson and Farmer (2013) in the Nile crocodile (Crocodylus niloticus).89 Thus, this feature would have to be considered original for archosaurs (Crocodilia, pterosaurs, dinosaurs, and birds). According to Farmer and Sanders (2010) studies, the data suggest that airflow is “extremely similar” to birds. However, it is unclear how it occurs without air sacs and by means of the diaphragm, and the mechanism remains to be determined (Farmer and Sanders 2010, 339, 340).90 Farmer (2015b) found bird-like flow-through breathing in the American alligator, two species of caiman, and three species of crocodile. This demonstrates that flow-through respiration is compatible with a pump-piston mechanism mediated by the liver (see Schachner et al. 2011, 1545).
Surprisingly, Schachner et al. (2014) also demonstrated an air circuit in the lungs of the steppe monitor (Varanus exanthematicus), which is even more distantly related to birds. It is therefore possible that this feature even connects all diapsids (snakes, lizards, crocodilia, and birds). However, according to these researchers, flow-through breathing could have evolved twice independently—in monitor lizards and archosaurs. To test this hypothesis, Cieri et al. (2014) studied respiration in the green iguana (Iguana iguana) and found flow-through respiration with simply constructed lungs in this species as well. This confirms the possibility that this trait may have already been evolved in Permian diapsids (see Farmer 2015a). However, according to Cieri et al. (2014), a convergent origin cannot be excluded. Further studies on respiration in different reptile groups are needed for clarification.
Evidence of the occurrence of flow-through respiration in various cold-blooded reptiles refutes the evolutionary hypothesis that this type of respiration arose from increased oxygen demand due to high metabolic outputs (Cieri et al. 2014). Hypothetical other selection pressures are discussed by Farmer (2015b).
Conclusion
Based on the widespread pneumatization of bones with air pores in theropod dinosaurs, there has long been considered strong evidence for at least partial avian-type respiration. This is also supported by recent findings of flow-through respiration in some present-day reptiles and the extensive study of theropod dinosaurs by Brocklehurst, Schachner, and Sellers (2018). More specific details cannot be inferred directly from the fossil record, as many avian details of the respiratory system are not fossil preservable and inferences from indirect evidence are fraught with uncertainty. However, the recently published study by Brocklehurst, Schachner, and Sellers (2018) seems to provide clarity that at least the osteological requirements for immobile lungs were present in Dinosauriformes, and thus bird-typical respiration was likely realized.
Whenever the origin of the avian respiratory and thus coupled air sac system is to be assumed under evolutionary theoretical conditions, the conversion is enormous due to the described interconnections (peculiarities in the construction of the lung, respiratory movements, coupling with thermoregulation) and it is unclear how it could have occurred in an evolutionary way. The few proposed remodeling steps (Farmer 2010; Sereno et al. 200891; Schachner et al. 2011; see figs. 33, 34) are very large and the models based on them do not take into account the numerous anatomical and physiological details.
Fig. 33. Phylogeny of the amniotes indicating the major changes associated with the respiratory system of birds. 1 Costal breathing and septate lungs, 2 four-chambered heart, unidirectionally ventilated lungs, 3 possible origin of postcranial pneumaticity and hypothesized associated air sacs, 4 dorsally immobile heterogeneously partitioned multichambered lungs with unidirectional airflow, possible thinning of the blood-gas barrier, 5 confirmed postcranial pulmonary pneumaticity, 6 hypothesized caudally positioned abdominal air sacs, 7 uncinate processes, 8 possible secondary loss of postcranial pulmonary pneumaticity in ornithischians (after Schachner et al. 2011).
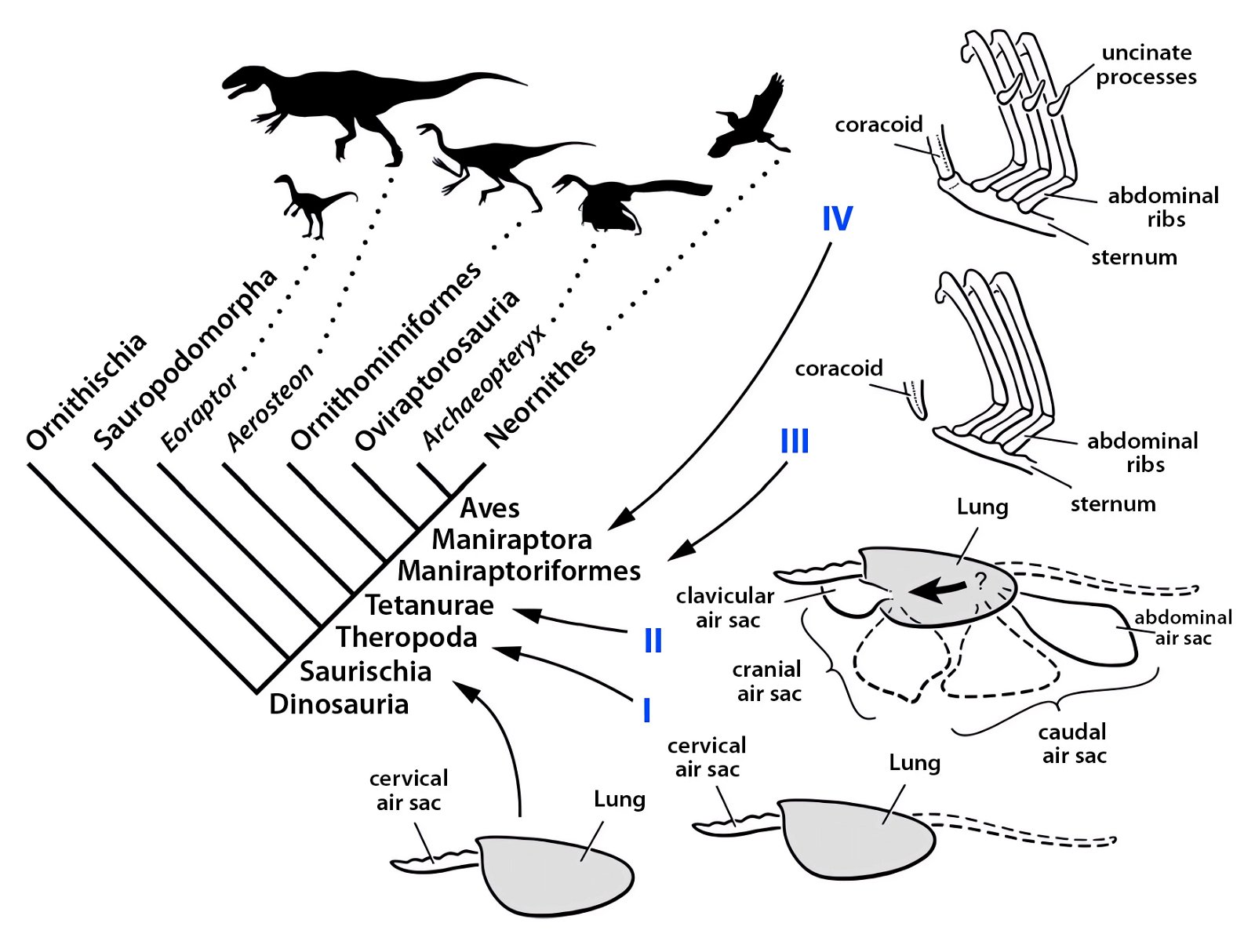
Fig. 34. Cladogram of dinosaurs showing a four-phase model for the evolution of air sacs and lung ventilation within theropods. Phase 1 (theropods): Variable posterior extension of paraxial cervical air sacs. Phase 2 (Tetanurae): air sacs in the clavicle and abdomen become divided and diverticula form under the skin. Stage 3 (Maniraptoriformes): simple respiration with the help of the ribs and sternum. Stage 4 (Maniraptora): advanced respiration using the ribs and sternum. The solid arrow on the lung indicates flow during pulmonary ventilation; the question mark indicates uncertainty about the direction of airflow (uni- or bidirectional) (adapted from Sereno et al. 2008.), CC BY-SA 2.5.
Usually, scientists refer to the “fact” that birds are a branch of theropods, so there must have been an evolutionary pathway for the respiratory system as well. Thus Witmer (2002, 21) writes: “Given the considerable evidence that birds are embedded within Theropoda, it would seem that indeed ‘you can get there from here,’ even if the physiological or anatomical mechanisms is at present obscure.” But this is a classic circular argument. The argument that for functional reasons a reconstruction seems to be impossible is covered up with reference to comparative biology or the cladistic classification. But the latter is only a method to create order, which serves as a basis for a phylogenetic interpretation. If a reconstruction were really functionally impossible, no counterargument could be developed from phylogenetic reconstructions.
Pelvis and Retroverted Pubis
The avian pelvis is characterized by strong fusions, which contributes to the lightweight structure of the skeleton. The last three thoracic vertebrae are fused with the lumbar vertebrae and some caudal vertebrae and together form the synsacrum, which in turn is strongly fused with the ilium. The ilium in turn is fused with the ischium and the anterior part of the pubis. The muscles of the legs and tail attach to the central platform thus formed (Proctor and Lynch 1993, 140). The long, thin pubis, which is fused to the ischium and abuts the ilium, is directed posteriorly (fig. 35).
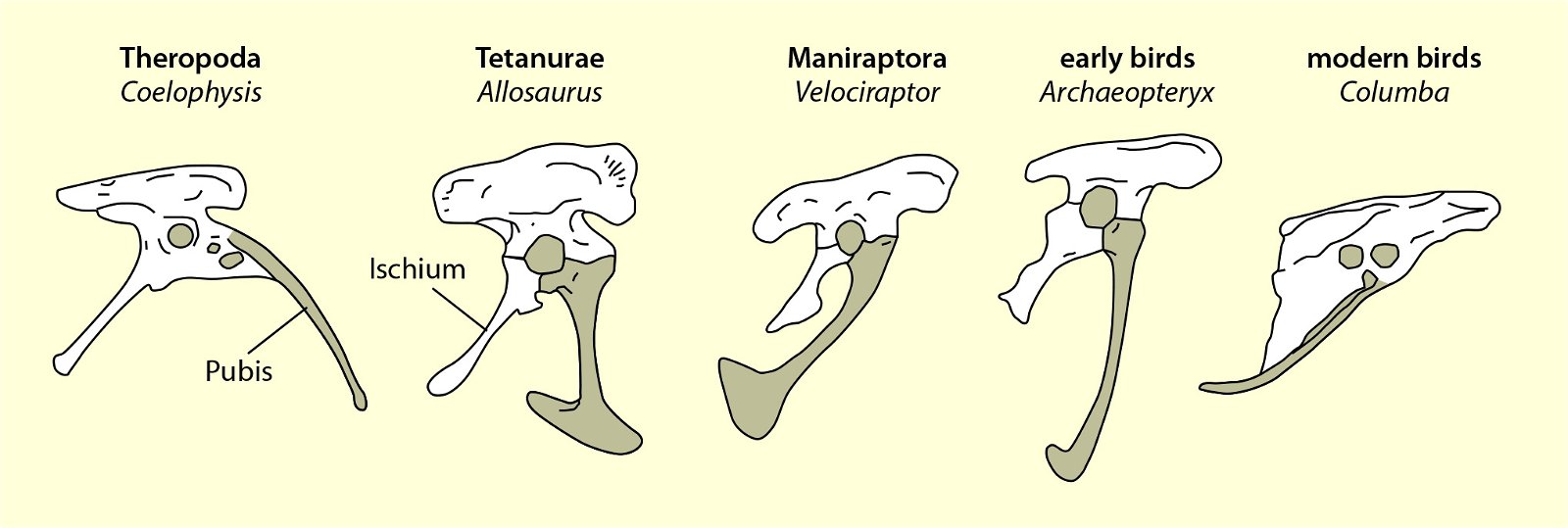
Fig. 35. Pelvic types in some theropods and birds (after Padian and Chiappe 1998a).
In most opposite birds and some ornithuromorphs, as well as in the basal genera Archaeopteryx, Jeholornis, Sapeornis, and Confuciusornis, the pelvic bones are not fused but are more or less clearly oriented posteriorly (Wang, Li, and Zhou 2017).92 Retroverted orientation of the pubis also occurs in many theropod dinosaurs, such as many dromaeosaurs (Elzanowski 2002, 15093; Norell and Makovicky 200494), some troodontids (Sinovenator, Xu et al. 2002b95), therizinosaurs (Clark, Maryañska, and Barsbold 2004), and in the derived genera of alvarezsaurids (Choiniere et al. 2014; Sereno 1997), but not in the upper Jurassic alvarezsaurid genus Haplocheirus (Choiniere et al. 2010). Since a retroverted orientation of the pubis is also absent in the basal genera of dromaeosaurs (Makovicky, Apesteguía, and Agnolín 200596) and most troodontids, a multiple convergent origin is assumed. Thus this characteristic cannot be evaluated convincingly as a “bird characteristic with dinosaurs”97, since it cannot have been developed under evolution-theoretical conditions continuously in the lineage leading to the birds. For example, Zhou (2004, 462f.) notes that, among other things, a retroverted pubis was developed in some theropods98 but not in the most basal birds.99 The same is true for Archaeopteryx (O’Connor, Chiappe, and Bell 2011, 45). With respect to this and other avian features, O’Connor, Chiappe and Bell (2011, 41) conclude that a highly homoplastic evolutionary history must be assumed based on their occurrence in apparently unrelated theropod groups and their absence in primitive birds (fig. 36).100
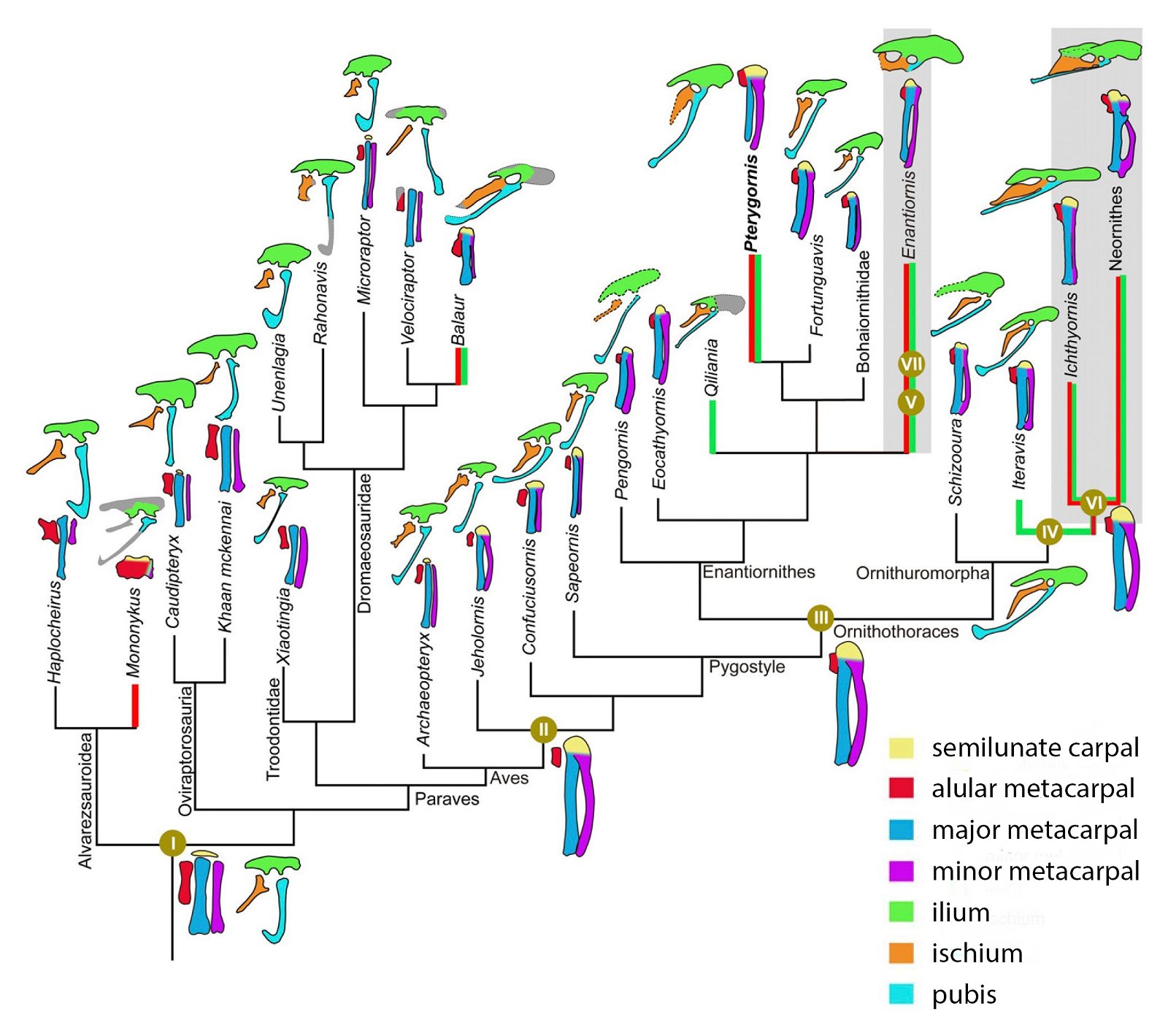
Fig. 36. Paravian phylogeny showing the major changes of manus and pelvis fusions. The ancestral conditions of the pelvis and manus fusions of the major nodes were reconstructed using the parsimonious method in Mesquite. Major changes are summarized below: I, the metacarpals and pelvis unfused in adults; II, the semilunate carpal fused with the proximal ends of the major and minor metacarpals, with the alular metacarpals separated; III, the alular metacarpal fused with the major metacarpal proximally but separated distally; IV and V, the ilium, ischium, and pubis fused around the acetabulum; VI and VII, alular and major metacarpals completely fused along their length. The green thick lines denote the taxa with fused pelvis, and the red thick lines indicate taxa with fused alular and major metacarpals. Late Cretaceous birds are denoted in shaded background. The line drawings are not to scale. It is clear that the fusions occur multiple times convergently and in incongruent distribution (from Wang, Li, and Zhou 2017. © 2017 National Academy of Sciences).
In the enigmatic scansoriopterygids, which in some respects are close to birds, the pubis is directed forward in a way that is untypical of birds (Czerkas n.d.), and in the fancy genus Yi from this group only little of the pelvis is preserved (Xu et al. 2015). Even in oviraptorids, which have a striking number of bird-typical features and are interpreted by some researchers as secondarily flightless birds (see below), the pubic bone was—as far as is known—directed anteriorly (Zhou et al. 2000, 252101) and the pelvic bones were not fused (Wang, Li, and Zhou 2017, 11474).
Fusion of the pelvic bones must also have occurred several times based on a current phylogeny, namely in the Upper Cretaceous dromaeosaur Balaur, in the enantiornithines Qiliania, Pterygornis, and Enantiornis, and in the ornithuromorphs, among which, however, not all genera possess fused pelvic bones (Wang, Li, and Zhou 2017, see fig. 36).102 According to Wang, Li, and Zhou (2017, 11474), a fused pelvis is also occasionally found in some ornithomimids, coelophysoids, and ceratosaurs. In summary, Wang, Li, and Zhou (2017, 11470) write that fusions in the pelvic region evolved independently in theropod dinosaurs, opposite birds, and ornithuromorphs. They say that fusions of these bones are rare in theropod dinosaurs and Lower Cretaceous birds, but well expressed in Upper Cretaceous and present-day birds. They show a complicated evolutionary pattern of different fusions.103
Carrano (2000, 489) notes that theropod dinosaurs have a remarkably uniform general pelvic girdle and hind limb morphology. There is little evidence of divergence to indicate the evolution of more specialized forms of locomotion, he adds. This apparent evolutionary stability makes the locomotor transition from theropod dinosaurs to birds even more remarkable. This transition involved changes in the entire locomotor system, not just the structures directly associated with flight.
Conclusion
Overall, it can be stated: There are enormous differences in the construction of the pelvis between birds known from Cretaceous strata and present-day birds. However, pelvic features typical of birds, such as backward orientation of the pubic bone and fusions in the pelvic region, which occur in theropod dinosaurs, can hardly be evaluated as bird features in dinosaurs, since an independent origin in the respective dinosaur groups is assumed due to the distribution in the system (fig. 37).
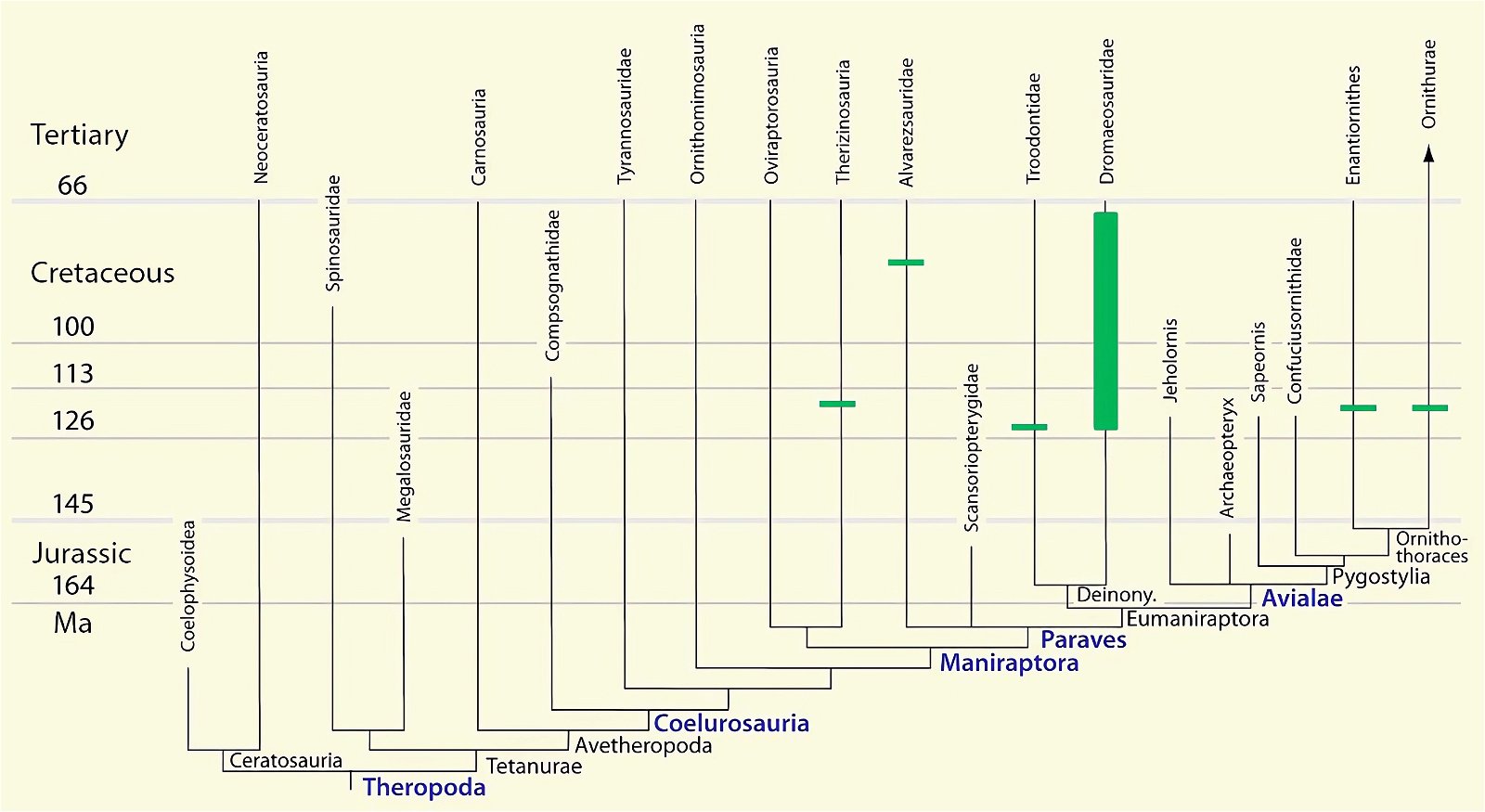
Fig. 37. Distributions of theropod and avian genera with retroverted pubes (green markings) (assembled according to the sources mentioned in the text).
Pygostyle
In today’s birds, the last tail vertebrae are fused to form a so-called pygostyle (fig. 38). It serves as a stable support for the fan-shaped tail feathers. The pygostyle is connected on both sides to a rectrical bulb, a complicated organ of fat, connective tissue and muscle (M. bulbi rectricium), to which the tail feathers attach and through which they can be moved and controlled. Only the two middle tail feathers are directly connected to the pygostyle.104 The associated ability to change the shape of the tail greatly enhances flight (Gatesy and Dial 1996, 2045ff; O’Connor et al. 2015a, 114). The whole system forms an intricate integrated unit and, together with the wings, enables the formation of a tightly coupled surface during flight (Gatesy and Dial 1996, 2037f).105 Wang and O’Connor (2017, 291) refer to this as an “elaborate tail complex” and an “integrated whole”. The uropygium (rear region) and integument (body covering) are morphologically interrelated, such that the shape of one can predict that of the other. This close interrelationship supports the evolutionary hypothesis that pygostyle and tail feathers co-evolve.106
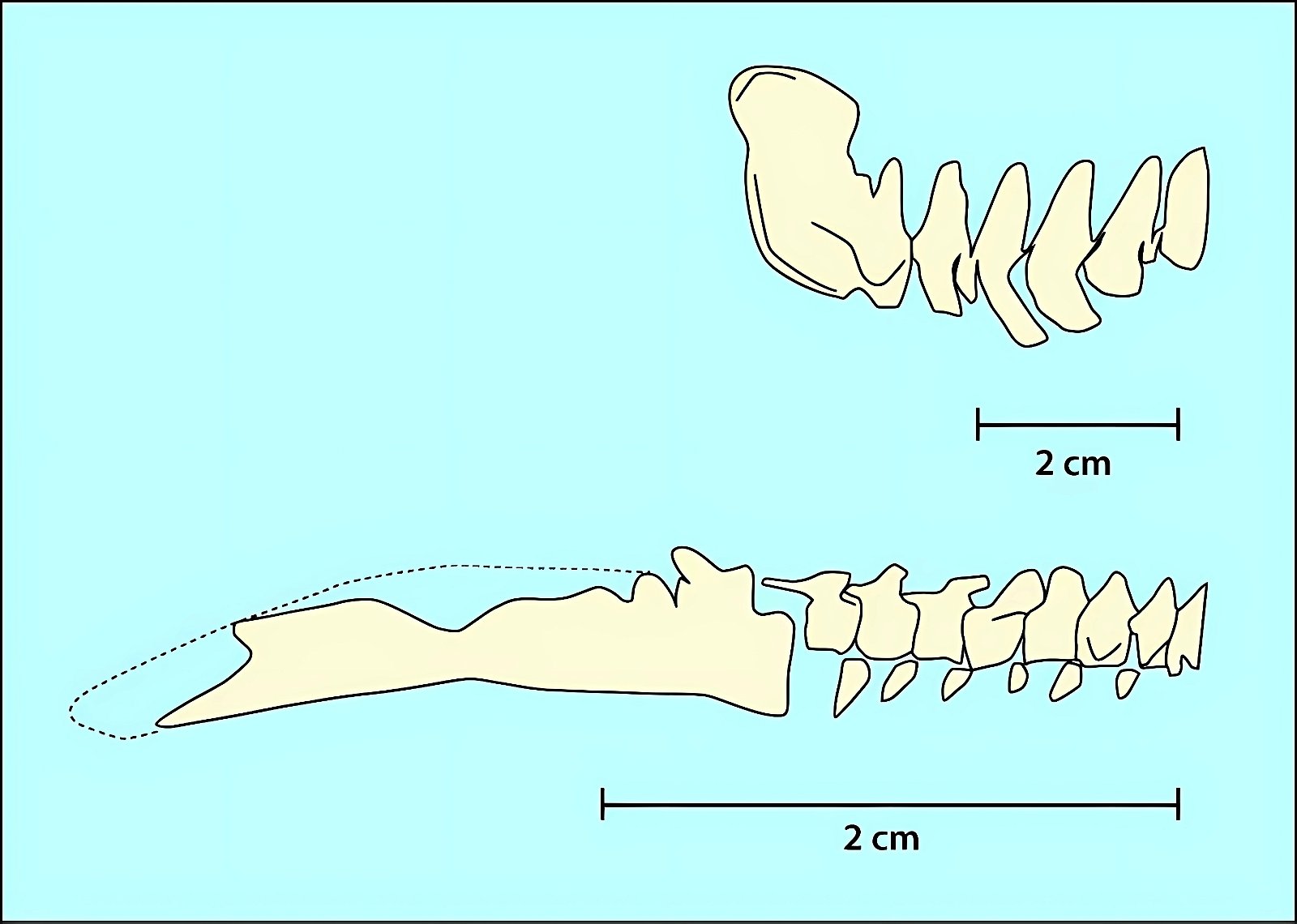
Fig. 38. Pygostyle of the pigeon (top) and the enantiornithine Iberomesornis (after Gatesy and Dial 1996).
A pygostyle is also developed in most fossil birds. They are grouped together as Pygostylia, although there are more marked differences in expression in basal species compared to present-day species. In contrast, the skeleton of the tail region of present-day birds is remarkably uniform despite diversity in tail expression.
Pygostyle Types in Fossil Forms
Three distinct pygostyle types survive among early birds fossilized in the Cretaceous geological system, only one of which occurs among modern birds (Hu, O’Connor, and Zhou 2015, 16f.; Wang and O’Connor 2017, 304107; figs. 39–42). The first type is short, plough-shaped, and tapering, occurring in Sapeornis and the Ornithuromorpha (for example, Archaeorhynchus, Yixianornis, Hongshanornis) and is similar to most Neornithes and thus to present-day birds.108 The second type is stout, forked, and constricted distally and is found in most Cretaceous enantiornithine birds. In Confuciusornis, a third type is developed; the pygostyle is stout and rod-like.
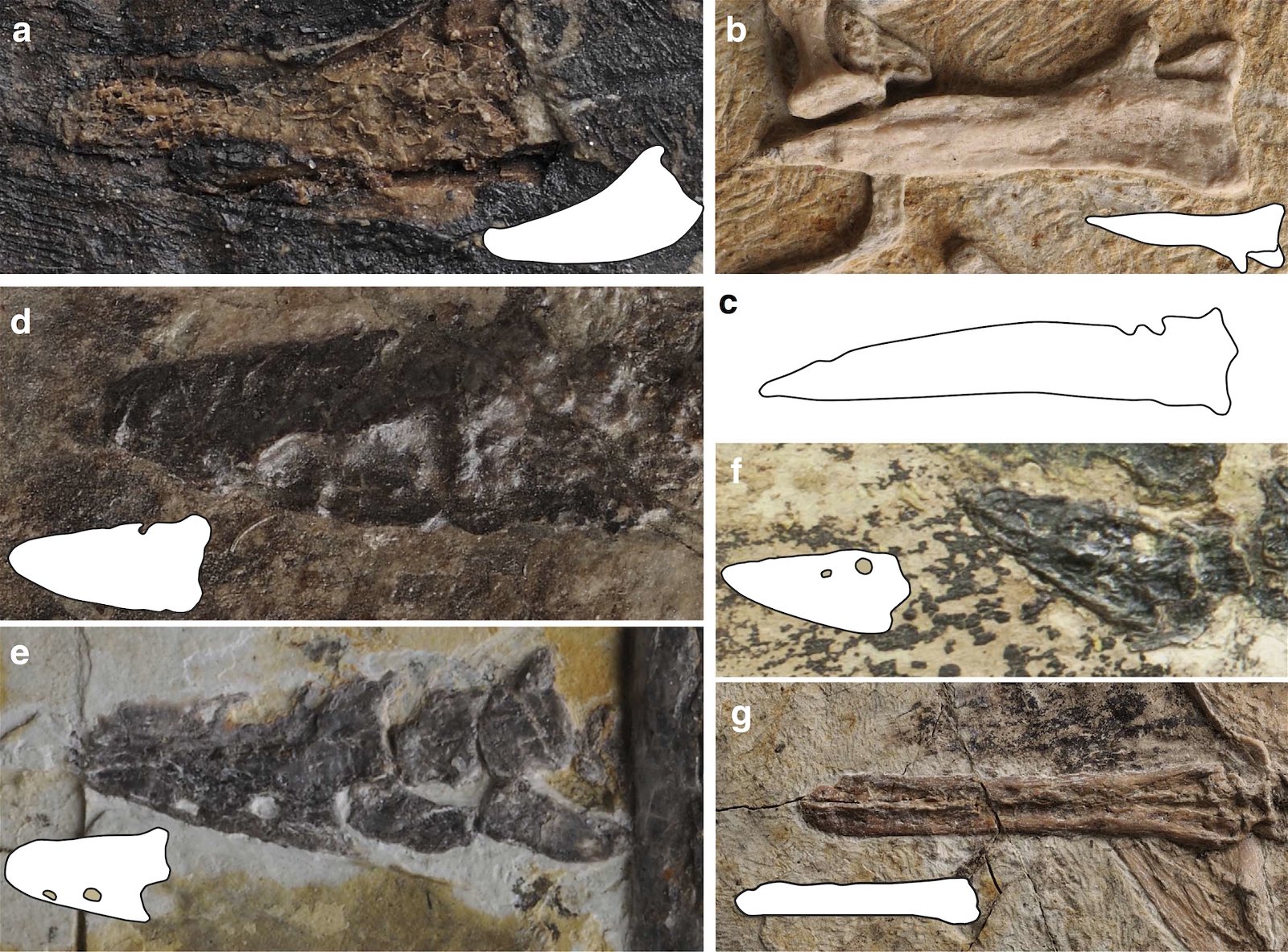
Fig. 39. Pygostyle of the enantiornithines Cruralispennia (a), Pterygornis (b), Concornis (e), the ornithurans Yixianornis (d), Bellulornis (e), Piscivoravis (f), and the basal bird Confuciusornis (g) (from Wang et al. 2017d), CC BY 4.0.
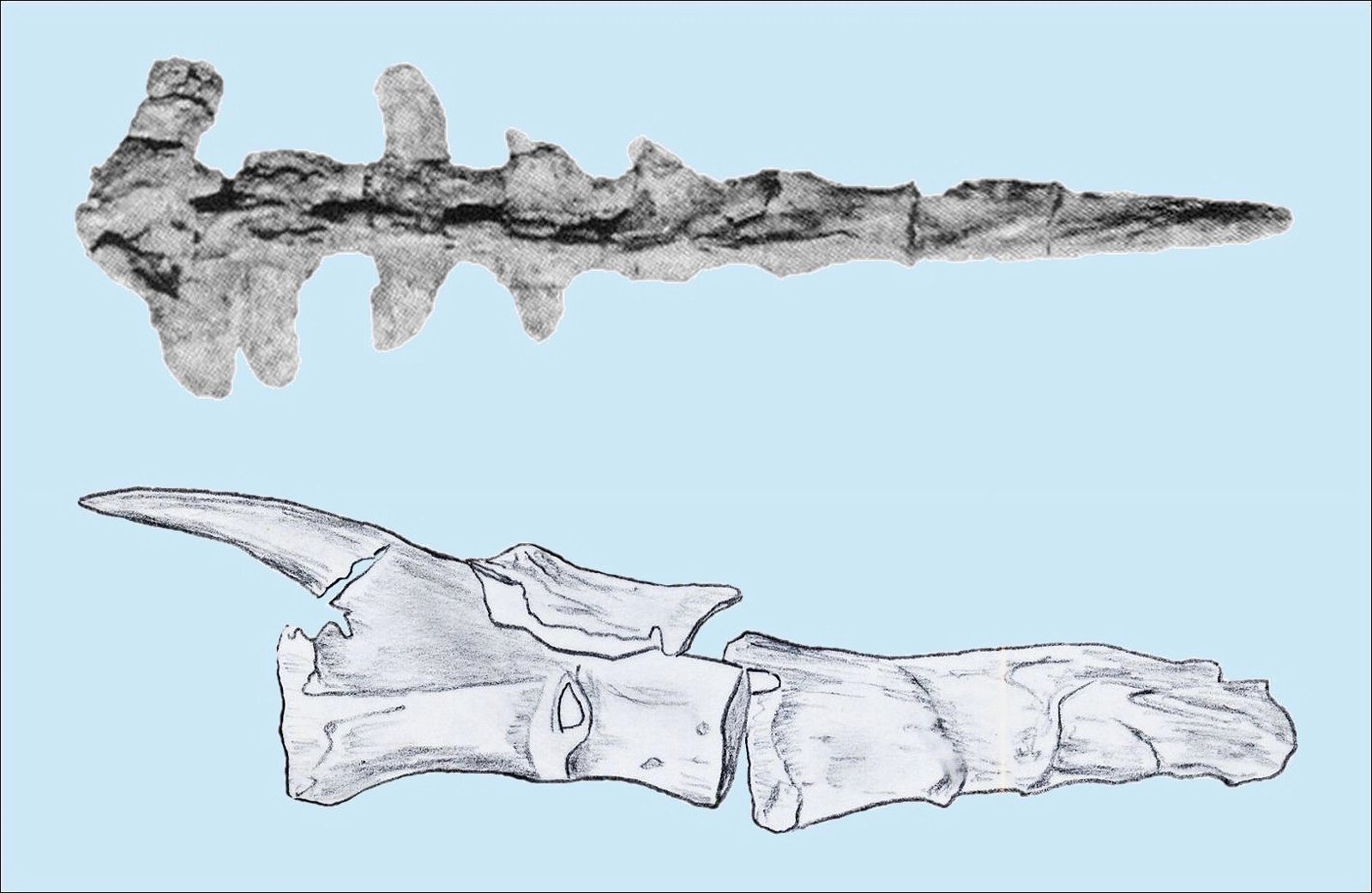
Fig. 40. Pygostyle of the oviraptorid Nomingia (from Barsbold et al. 2000b). CC BY 2-0). Pygostyle of the therizinosaur Beipiaosaurus (after Xu et al. 2003a).
Fig. 41. Epidexipteryx with elongate pygostyle. Jaime A. Headden, http://qilong.deviantart.com/art/Nitpicker-103918005/, CC BY 3.0. License (original).
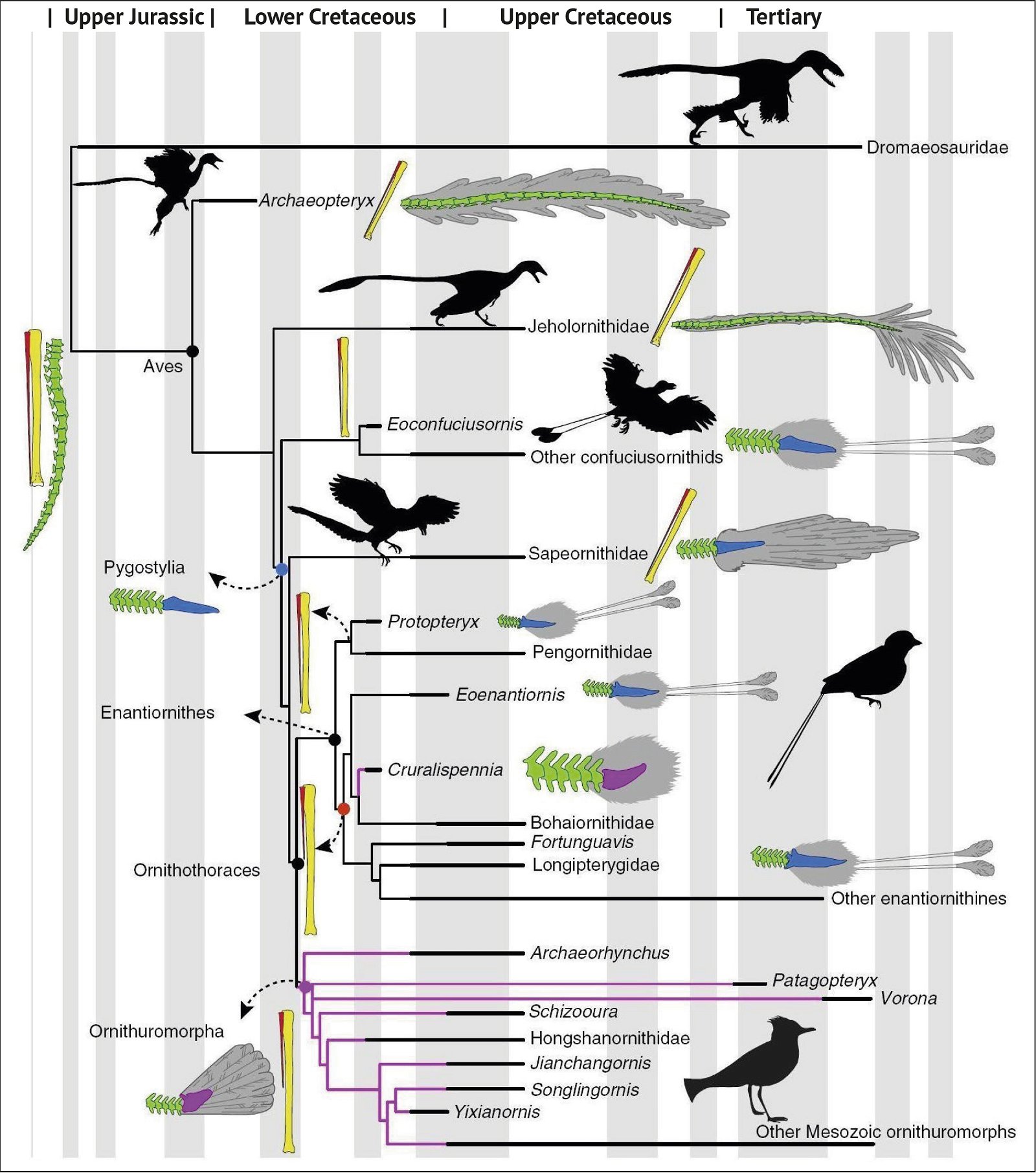
Fig. 42. Fibula and tail morphology (pygostyle) in Mesozoic birds entered into the consensus cladogram after Wang et al. (2017d). The thick lines indicate the temporal range of fossil taxa. An elongate fibula (red) is developed in basal birds (outside the Ornithothoraces) and in the basal enantiornithines Protopteryx and the Pengornithidae; a reduced fibula is convergently realized in ornithuromorphs and derived enantiornithines. The enantiornithine Cruralispennia has a plough-shaped pygostyle (pink) similar in shape to ornithurans (pink branches) (from Wang et al. 2017d), CC BY 4.0.
It is possible that the pygostyles of the second and third types (in Confuciusornis and also in oviraptorosaurids) only allowed for weight savings without playing any particular role in flight performance (Clarke, Zhou, and Zhang 2006).
In contrast, the bipedal theropods generally possessed long tails with dozens of free caudal vertebrae, which presumably served for balance. Robust transverse projections on the anterior caudal vertebrae served as attachment points for the large caudofemoralis muscles, which primarily served as retractors of the hind legs (Rashid et al. 2014, 2).109 The two tail types—pinnate tail and fan tail—are functionally very different. The hind legs were functionally linked to the long tail (Benson and Choiniere 2013, 1), and to evolve the fan tail, this functional coupling had to be removed, further emphasizing that the transition to a fan tail required significant modification. However, some genera of theropod dinosaurs also possessed a pygostyle (see below).
Distribution
Birds with long “dinosaur tails” (Jeholornis and related species, Microraptor, and Archaeopteryx) and species with pygostyles and fan tails coexisted side by side (Brusatte, O’Connor, and Jarvis 2015, 290; Rashid et al. 2018, 8). Many maniraptorans (with long tails), considered precursor groups of birds, are stratigraphically younger (in some cases significantly so) than numerous Pygostylia. Species with pygostyles or pygostyle-like structures are distributed on different branches in the theropod cladogram in such a way that multiple independent (convergent) origins must be assumed (for Oviraptorosauria and Therizinosauria: Kurochkin 2006110, Barsbold et al. 2000a111, b [Nomingia], O’Connor and Sullivan 2014; Persons, Currie, and Norell 2014 [Nomingia, Citipati, Conchoraptor], Xu et al. 2003a [Beipiaosaurus]; there is also another convergent tail shortening in the scansoriopterygid Epidexipteryx; Rashid et al. 2014, 3; Wang and O’Connor 2017112; figs. 41–43).
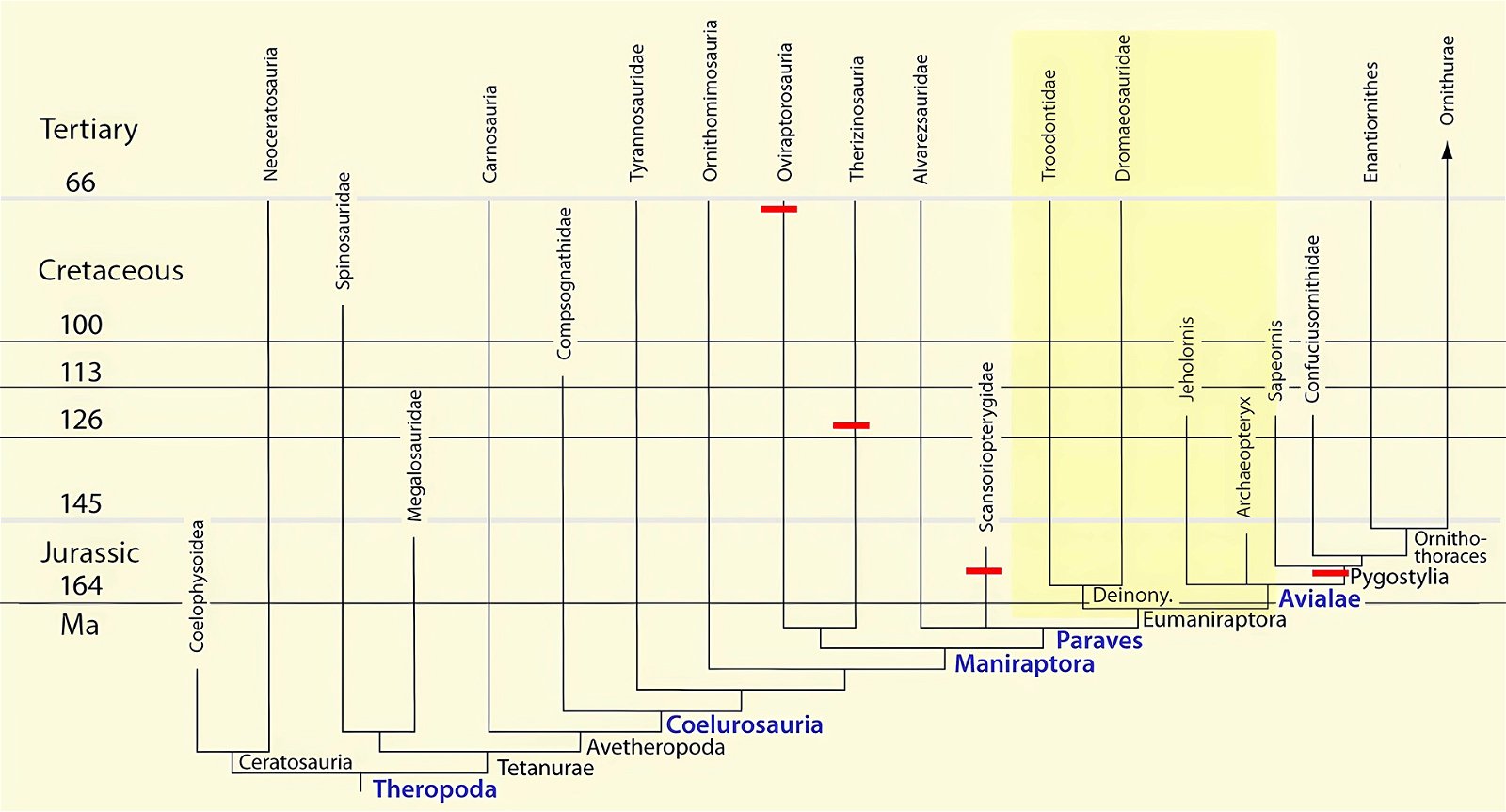
Fig. 43. Distributions of theropod and bird genera with pygostyle (red markings) (assembled according to the sources mentioned in the text).
The different pygostyle types are very likely functionally dissimilar, as mentioned earlier. Wang and O’Connor (2017, 303) consider it unlikely, based on the structure of the pygostyle in oviraptorosaurs, that a rectricial bulb or equivalent structures were developed.113 Persons, Currie and Norell (2014, 553, 562, 564) believe that the tail region of Oviraptorosauria was unique, did not fit into a transitional position to an avian pygostyle, but was a convergent formation, and most likely had a scooping function.114 That the different pygostyles must be interpreted as convergent also follows from their distribution in the cladogram (see above), unless one interprets Oviraptorosauria as secondarily flightless birds (see below and Mayr 2017a, 45115).
Transitional Forms?
Wang and O’Connor (2017, 289) refer to the emergence of the pygostyle and the fan-shaped arrangement of feathers as one of the “most remarkable adaptations” of early avian evolution, but a gradual transition between forms without and with a pygostyle is not fossil documented (also O’Connor et al. 2015a, 114116; Mayr 2017a, 45117). Wang and Zhou (2017, 6) also note that little is known about how the shortening of the tail occurred, as relevant fossils documenting this transition are lacking.118 The oldest pygostyles of all Lower Cretaceous ornithuromorphs are nearly “modern” in form. Together with the occurrence of fan-shaped tail feathers, this argues, according to Wang and O’Connor (2017, 289, 305), that the complete avian tail complex evolved very early in this lineage and that the rectricial bulbs and fan tail evolved together.119 O’Connor et al. (2015a, 114) also argue along these lines: The co-occurrence of a pygostyle and aerodynamic tail fans in ornithurans, Sapeornithiformes120, and pengornithids121, a basal group of the opposite birds, supports coevolution of the entire tail complex. It is the most parsimonious explanation, he argues, that the rectricial bulb was lost in Confuciusornis relatives and some opposite birds.122 In contrast, O’Connor et al. (2015a, 117) argue that it is incomprehensible that the derived groups of the opposite birds would have abandoned this complex again in favor of a more robust pygostyle and argue for an independent origin of pygostyles with the rectricial bulb. This is also supported by the fact that there are morphological differences in pygostyles.123
Zhongornis haoae (a juvenile species of unclear systematic position) was discussed as an intermediate form, but a reexamination of the tail did not confirm this (O’Connor and Sullivan 2014). Rather, about 20 caudal vertebrae are preserved, although the actual number may be even higher (Archaeopteryx had 23 caudal vertebrae). The tail is said to be very similar to that of Epidexipteryx (Scansoriopterygidae) and Caudipteryx (Oviraptorosauria). However, a recent study by Rashid et al. (2018) showed that fusion of the outermost caudal vertebrae into a pygostyle occurs just before skeletal maturation in present-day birds. Therefore, it should be assumed that in the juvenile fossil of Zhongornis, the pygostyle was not yet mature.124
That the differences between both tail types are significant, Gatesy and Dial (1996, 2046) also note, as well as that the evolutionary bridging is rather weak. Although Iberomesornis has already been mentioned here, this genus is clearly in the direction of present-day birds, however (fig. 38 clearly shows this). It is added that the pygostyle in Iberomesornis is fused from 10–15 vertebrae; otherwise, it is only 6–8. This situation does not fit to an intermediate form.
The genus Cruralispennia is noteworthy. Although it belongs to the opposite birds, the shape of its pygostyle tends rather to the ornithuromorphs and is clearly different from pygostyles of the opposite birds (Wang et al. 2017d; fig. 39). Thus, it is a mosaic form that can only be phylogenetically classified assuming convergence.
Rapid Emergence of the Pygostyle?
Could one or could very few mutations have caused the bird-like situation of the tail region and therefore lack transitional forms? Rashid et al. (2014, 2018) think so. They argue that the reduction in the number of caudal vertebrae and the emergence of the pygostyle occurred in a “very short evolutionary interval.” They argue that the features of the tail complex occur in a coupled fashion (plus other features125), so that one mutation could lead to multiple changes simultaneously. Experiments with mice, they argue, have shown that mutations can lead to fusions of vertebrae and tail shortening and have pleiotropic effects (that is, have multiple effects simultaneously). Accordingly, a few changes from Jeholornis could have led to Confuciusornis: “If a vertebral fusion mutation occurred in a primitive bird like Jeholornis, which fused additional vertebrae in its synsacrum, truncated its tail, and fused some ribs, the resulting creature would have come a long way toward resembling Confuciusornis” (Rashid et al. 2014, 16).
Underlying this argument, however, is the common fallacy of confusing a necessary condition (here, changes in a regulatory gene) with a sufficient explanation. Especially the complexity and integration of the tail region of birds would certainly require numerous changes in downstream areas and tuning of interactions. One or a few regulatory mutations are far from enough. Mutations that lead to fusions are most likely to be severely detrimental on their own and have negative side effects (the abovementioned mice are certainly not selectively advantaged, if they are viable at all). Matching changes in muscle and other tissues would have to occur simultaneously, which is not likely. A scenario of a sudden, large-scale change that would have to involve many matching tunings is an extremely unlikely speculation, even in the age of EvoDevo.
Conclusion
The pygostyle does not belong to the bird-typical structures that supposedly have already evolved in dinosaur-like bird precursors. There is of course (in evolutionary theoretical interpretation) a convergent (independent) origination of pygostyles in younger forms of theropod dinosaurs. However, these are only fossilised well after the appearance of the first birds (fig. 43). Consequently they cannot be interpreted as bird precursors. Various pygostyle types are known, including such not found among present-day birds, but forms with and without pygostyles (and consequently with fan tails and plumage tails, respectively) are clearly separated. There are, however, some bird genera with a pinnate tail, namely Jeholornis and Archaeopteryx, and the four-winged Microraptor.
Reduced Fibula
The fibula in birds is greatly reduced, shorter than the tibia and formed as a kind of bone brace, which helps to save weight. Two tarsal bones are fused with the tibia to form the tibiotarsus.
In contrast, in many Mesozoic birds, the fibula is as long as the tibia.126 This is also the case in theropod dinosaurs, in which, however, the fibula is narrow and in close contact with the tibia (Botelho et al. 2016, 543). The stratigraphically oldest bird genus with a reduced fibula is Cruralispennia from the enantiornithine group. At 131 Ma, this genus is also among the geologically oldest opposite birds (Wang et al. 2017d).
The distribution of genera with reduced tibiae in the system of theropods and birds requires the assumption of multiple independent regressions, namely in the Alvarezsauridae, Oviraptorosauria, and the birds, furthermore, also in the pterosaurs. Moreover, it must be assumed that the reduction in the size ratio of the fibula and tibia occurred independently in different avian lineages (as in Confuciusornis, Quiliania, and Gansus) (Altangerel et al. 1993; Botelho et al. 2016, 543; Makovicky and Zanno 2011; Sereno 1997; Wang et al. 2017d, 8127; see figs. 42, 44, 45). There is no consensus in the scientific discussion as to whether the reduction of the fibula should rather be considered an evolutionary by-product or an adaptive structure (Botelho et al. 2016, 551f.).
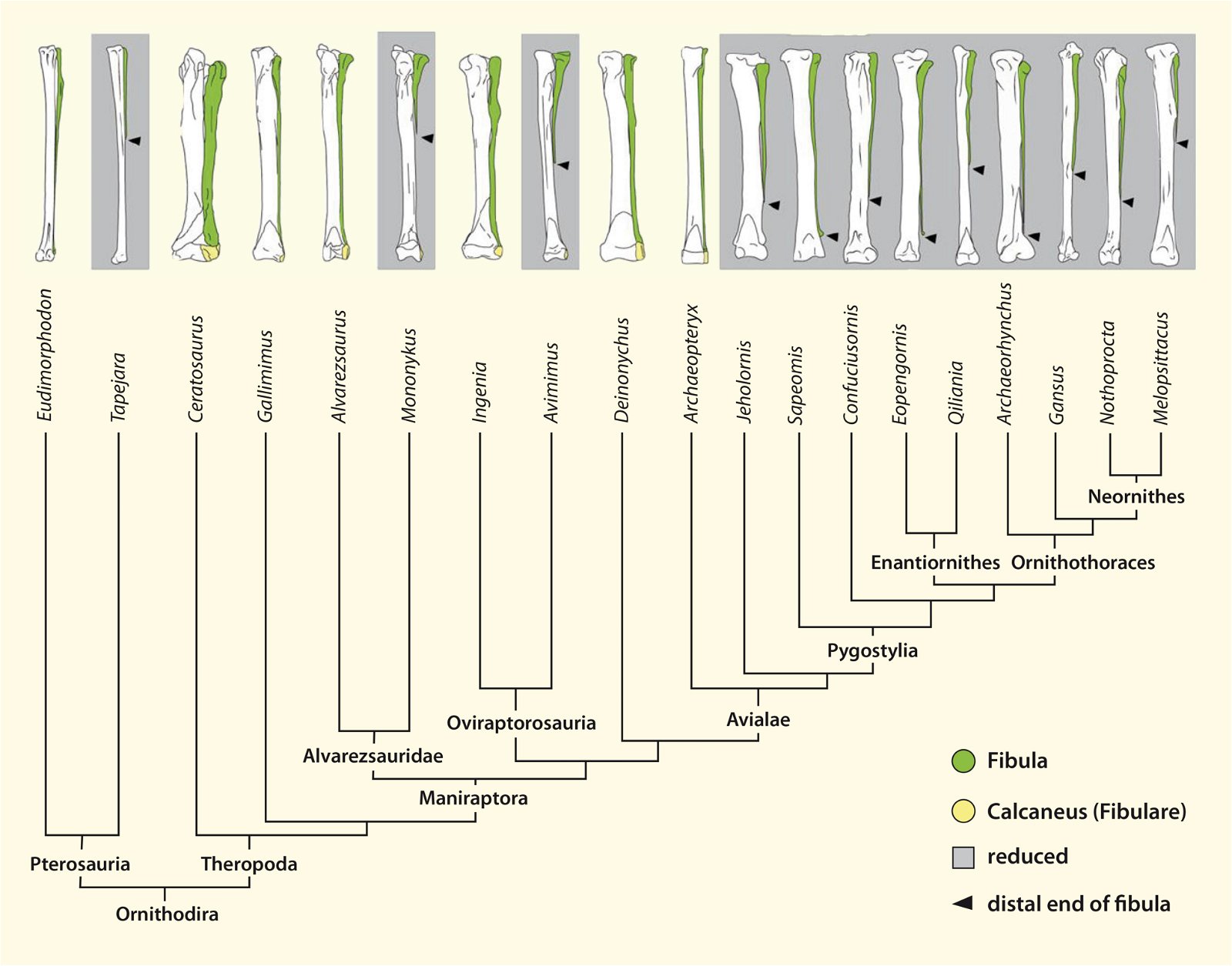
Fig. 44. Convergent reduction of the distal fibula in Ornithodira (pterosaurs and dinosaurs). At its base is a slender fibula with reduced distance between it and the tibia. Based on the distribution in the system, evolutionary theory must assume a reduction of the fibula independently (convergent) in four clades: Pterosauria, Alvarezsauridae, Oviraptorosauria, and birds. In basal Mesozoic birds such as Jeholornis, Sapeornis, Eopengornis, and Archaeorhynchus, the fibula was already formed distally in a splinter-like fashion without a distal epiphysis. However, the fibula was only slightly shorter than the tibia. A low size ratio of fibula and tibia is also convergently realized in Confuciusornis, Qiliania, and Gansus (adapted from Botelho et al. 2016, CC BY 4.0).
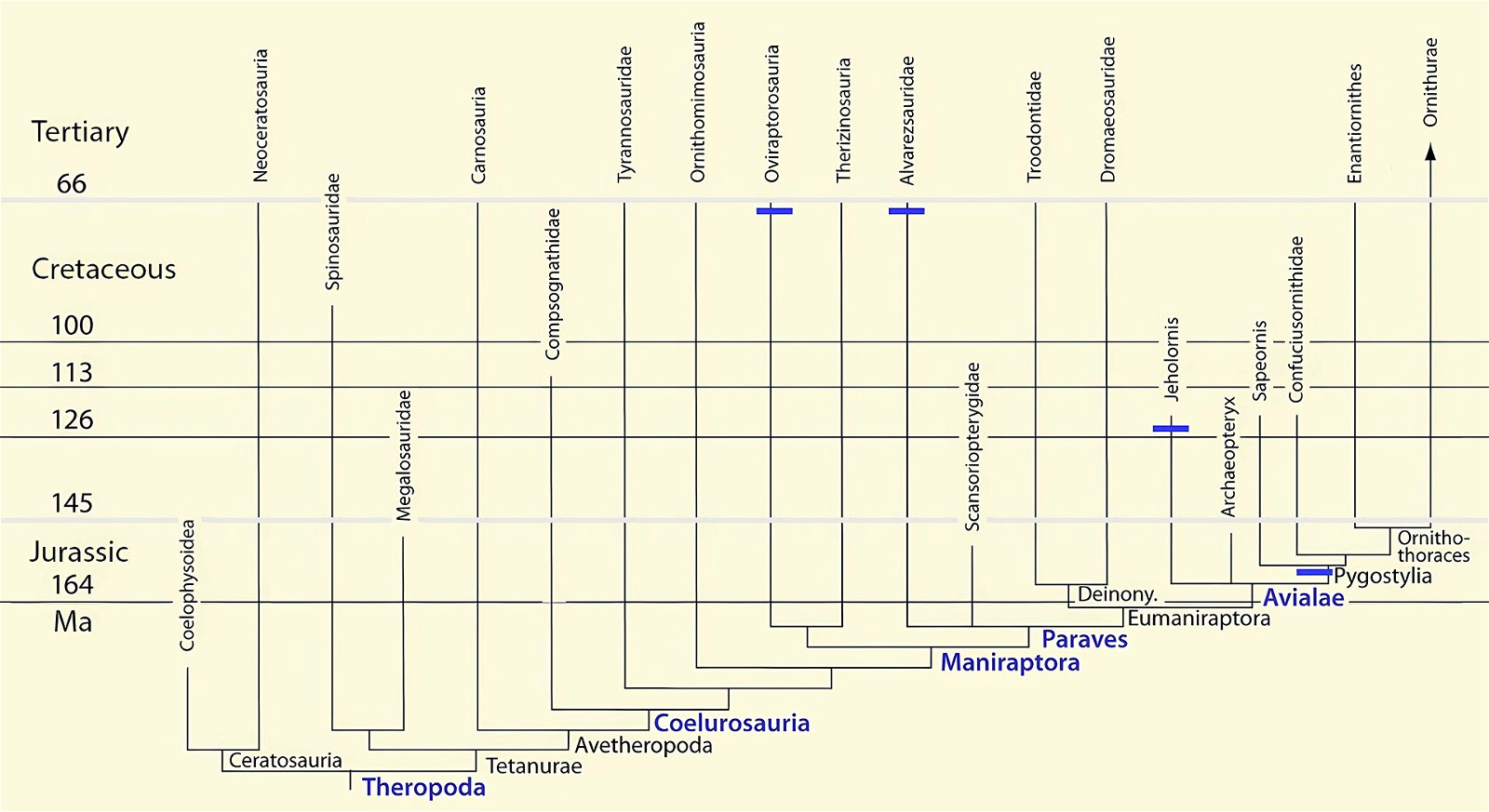
Fig. 45. Distributions of theropod and avian genera with reduced fibulae (blue markings) (assembled according to the sources mentioned in the text).
Botelho et al. (2016) experimentally manipulated tibia growth in birds and, based on their results, hypothesized that fibula reduction occurred phylogenetically in two steps: first, the loss of the fibula’s connection to the ankle, and then its reduction. Thus, both hypothetical processes would have to have occurred independently several times.
Wrist with a Semilunate Carpal
The mobility of the wrist is unique in birds because birds can angle the hand against the ulnar side of the forearm (that is, toward the ulna) and other angles are nearly impossible (Peters 1985, 244). This is made possible by the special construction of the wrist. It has a semilunate carpal bone (carpal, radiale), which faces the distinctly convex trochlea (articular surface) at the anterior end of the carpometacarpus and is important for the strong mobility of the wrist during flight and when folding the wings on the ground (Sullivan et al. 2010, 2027; Vasquez 1992). A semilunate carpal is also found in numerous dinosaurs and characterizes a broad taxon, the Tetanurae (Witmer 2002, 20; Norell and Makovicky 1999, 38128; see. fig. 46). In the three-fingered predatory dinosaurs, its function is unclear. Presumably it enabled rotational movements in grasping and manipulating prey (Sullivan et al. 2010, 2031129) or, in feathered forms, enabled better protection of the pennibrachium (in coelurosaurs, term for wing-like forelimbs with feathers with which a flight presumably was not possible) (Sullivan et al. 2010, 2032130). In Archaeopteryx and later birds, it enabled the bird-like rotations in wing flapping.131 It is commonly assumed in evolutionary theory that the possession of a semilunate carpal was a kind of preadaptation for the requirements of flight and could therefore be coopted for flight.
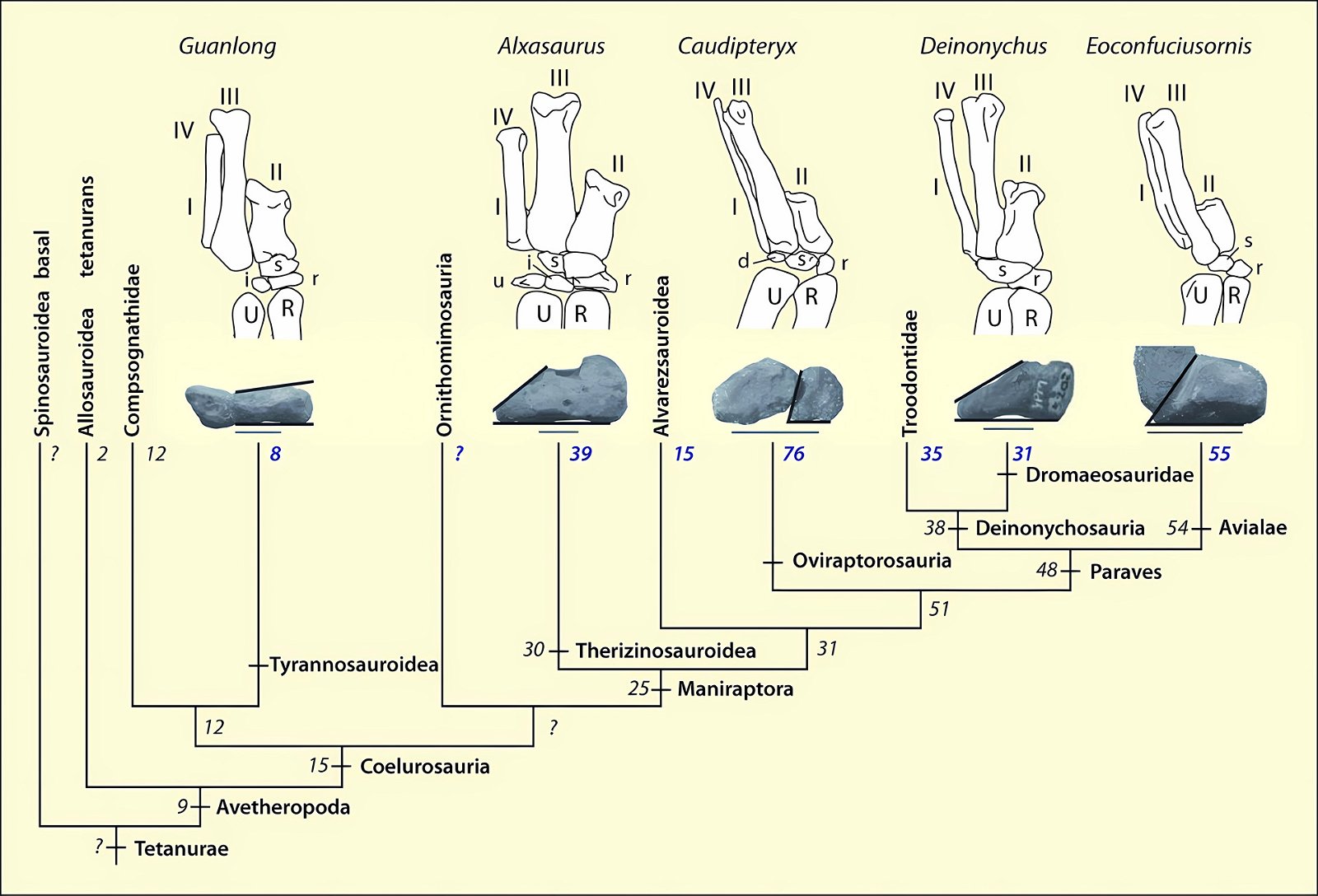
Fig. 46. Wrist structure and radial angle in tetanurans (phylogeny after Smith et al. [2007] and Zanno et al. [2009]). Figures indicate values of radial angle between proximal and distal articular surfaces of the radials in degrees. Values in bold are direct measurements of individual taxa; values in normal italic font are reconstructed ancestral states. II–IV metacarpals II–IV (numbering follows situation in present-day birds), d distal carpals, i intermedium, R radius (radius), r radials. s semilunate carpals, U ulna (ulna); u ulnar. Scale bars: 0.25 cm in Eoconfuciusornis, 0.5 cm in Caudipteryx, 1 cm in all other taxa (after Sullivan et al. 2010).
The wrist bone structure is very similar in all present-day flight-capable birds, despite very different ecologies and lifestyles. This is strong evidence that this bone structure is necessary for flapping flight (Vasquez 1992, 266). In Archaeopteryx and theropod dinosaurs, ulnar angulation was probably not as pronounced as in other Mesozoic and present-day birds (Sullivan et al. 2010, 2027132), and the wrist of Archaeopteryx is not considered capable of sophisticated ground takeoff and active flight abilities comparable to present-day birds (Vazquez 1992133; Chatterjee and Templin 2003, 28134).
Partially Problematic Homologies
The homology of the semilunate carpal in birds and dinosaurs is not without controversy: “It is possible that the SLC [semilunate carpal] is not homologous throughout Tetanurae, in that different distal carpal elements may contribute to forming the SLC in different taxa or at least contribute to varying degrees” (Sullivan et al. 2010, 2030).
Based on their analyses, Xu et al. (2014, 1) conclude that the semilunate carpal was not formed by the same carpal bones in all theropods. They postulate a homeotic transformation that led to a lateral shift in position during theropod evolution.135 Curiously, they note that this transformation may have been selected for the purpose of folding wings (“result of selection for foldable wings”), which implies purposefulness, since this process is supposed to have occurred before wings existed. The assumption of a homeotic transformation seems to be an ad hoc explanation for which there is no evidence.136
An evolutionary sequence proves to be even more problematic based on a study by Botelho et al. (2014). A synopsis of embryological and paleontological data led these authors to conclude that there had been a remarkable evolutionary regression, namely, a large bony pisiform (one of the carpal bones) had initially regressed during evolution, but then reappeared fully formed. In the meantime, it must have either disappeared, or it was not ossified, or it was very small.137 In contrast, fusions could plausibly be made in other bone elements of the hand (Botelho et al. 2014, 2). These authors also argue a purpose (which should be taboo for evolutionary explanations): “The purpose of the re-evolution of the pisiform was probably to facilitate flight” (Botelho et al. 2014, 2).138 Such a scenario contradicts Dollo’s law of non-reevolution of more complex structures. Apparently, however, such a process is not “forbidden,” so examples like this only show that there are no laws of (innovative) evolution.
Larry Martin, one of the critics of the dinosaur-bird theory, believes that the maniraptorans are more interpretable as descendants than ancestors of birds, given their hand characteristics, because the wrist is less derived in some respects in Archaeopteryx and modern birds (Martin 2004, 981).
No Continuous Change
The angle between the proximal and distal articular surfaces is called the “radial angle” and is thought to have increased in coelurosaurs and especially in maniraptorans. However, this increase is far from continuous, as can be seen from the figures in fig. 46. Accordingly, there is a jump in Therizinosauroidea from 12° to 39°, then in Alvarezsaurids a decrease to 15°, while in oviraptorosaurids 76° and in Troodontids 35° were determined. Sullivan et al. (2010, 2031) therefore consider that the wrist of oviraptorosaurs evolved independently from that of birds. Fig. 47 shows the situation of the partly unsystematic distribution of trait expressions of further traits related to the wrist.
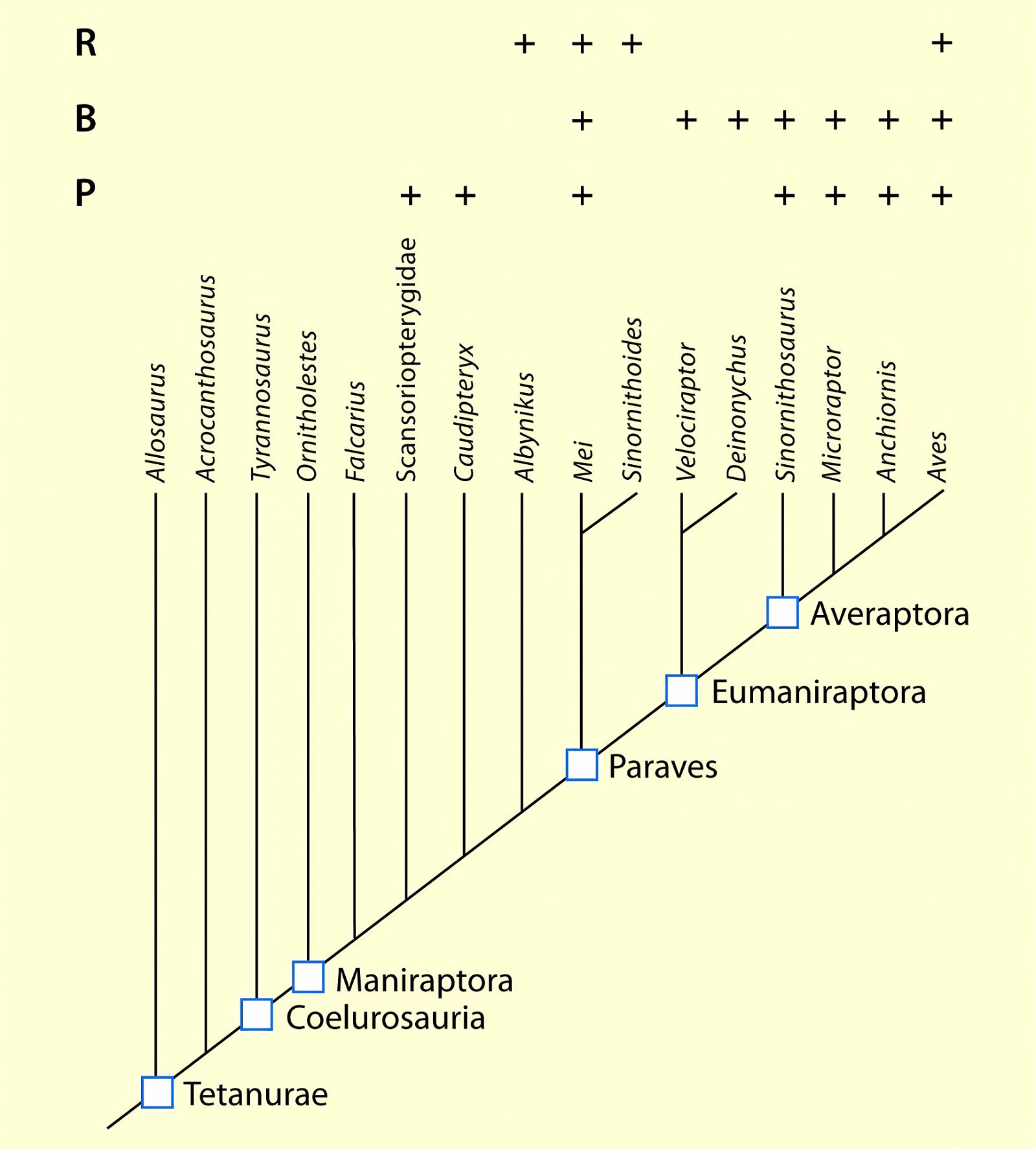
Fig. 47. Simplified cladogram of Tetanurae (see also fig. 4) showing the distribution of the following features: R bird-like resting position of the wings, B good flexion ability of the wrist, P propatagium (anterior flight skin) (according to Agnolin et al. 2019.
Another problem for the idea of a more or less continuous change towards bird-like mobility of the wrist is the fact that the restriction to ulnar abduction would be distinctly unfavorable for both a runner and a climber, but favorable for flying (Peters 2002, 350). This implies, however, that corresponding non-flying antecedent forms would have to be distinctly different from birds on functional grounds, arguing against a gradual transition. Climbing ability and flying ability of the anterior extremity contradict each other (Peters 1994, 406; see Peters 1985, 245).
Conclusion
The widespread formation of a semilunate carpal in numerous theropod dinosaurs could be considered a type of preadaptation for the requirements of flight. However, the homology of the semilunate carpal in birds and dinosaurs and within dinosaur groups is controversial. Evolutionary theory must assume that a large pisiform bone initially regressed, but then became fully developed again. In contrast, fusions can be made plausible for other bony elements of the hand.
Eggs, Clutches, and Brood Care
Most modern birds build nests and practice pronounced brood care. Females lay large eggs relative to body size and only one ovary is developed (the left one), not two as in reptiles. Accordingly, only one egg is laid in a single laying. In many species both partners breed, in some only the female, rarely exclusively the male. The eggs are turned frequently to ensure even warming. The chalky part of the eggshell has a three-layer structure (Hincke et al. 2012). The cuticle is first followed by a layer of vertically arranged crystals, then comes a palisade layer (with prismatic or scale-like elements) and finally a layer of cone-shaped components (mammillary layer), which is abutted by two membrane layers inside the shell (see figs. 48, 49).139
Fig. 48. Structure of an eggshell of modern birds (from Hincke et al. 2012).
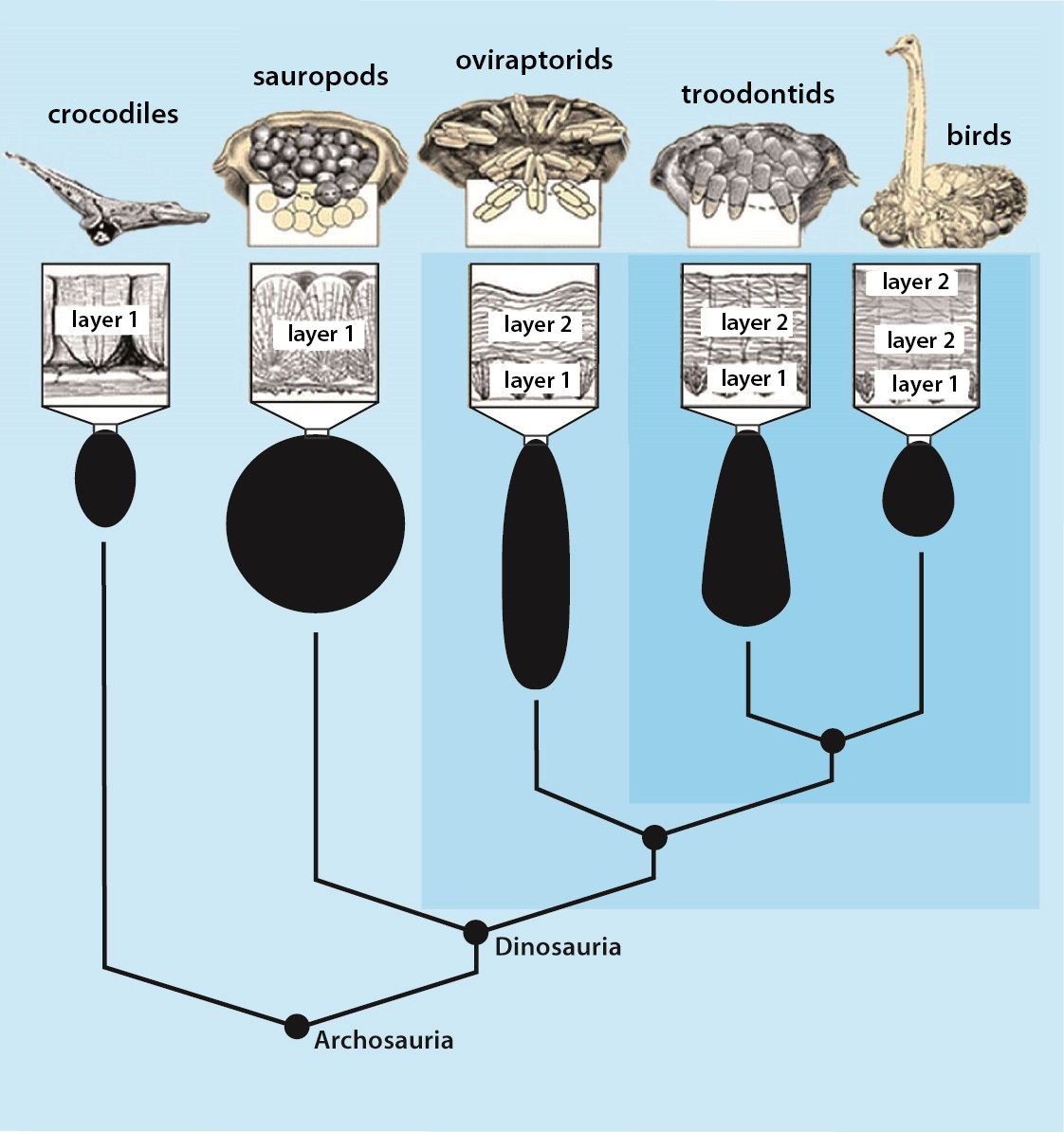
Fig. 49. Characteristics of the eggs and clutches of crocodiles, some dinosaurs, and birds. For example, the presence of at least two crystalline layers in the eggshell and the existence of an asymmetric egg can be traced back to the base of the maniraptorans (Jackson, Horner, and Varricchio (2010) were even able to confirm that the eggshells of Troodon were three-layered (see also Varricchio et al. 1997)). The distribution of eggs within a clutch in oviraptorids suggests that these dinosaurs laid their eggs sequentially. There is also evidence that, like birds, they also brooded their clutches (according to Chiappe 2009).
A number of bird-like features in egg structure and brood care, as well as bird-like nesting behavior, are also found in theropod dinosaurs, especially oviraptorids (Norell et al. 1995140; Zelenitsky and Therrien 2008), but also in troodontids (Varricchio et al. 1997; Varricchio and Jackson 2004a; Varricchio, Kundrát and Hogan 2018; Zelenitsky and Therrien 2008). Eggs with two-layered shells were found in a fossil of the dromaeosaur Deinonychus (Grellet-Tinner and Makovicky 2006141) and embryos in eggs were fossil preserved in a therizinosaur (Kundrát et al. 2008). In addition, incomplete, three-layered eggshells and two eggs and several other clusters of shell fragments of an alvarezsaurid (Bonapartenykus) were discovered in strata of the Upper Cretaceous of Argentina (Agnolin et al. 2012). The shape of the eggs could not be reconstructed here. The pairing of the fossil eggs may indicate that there were two functional oviducts. More detailed information about nests or brood care is not known for Deinonychus, Bonapartenykus142 and the mentioned therizinosaur with the fossil embryos in the eggs.
The accumulation of finds of nests with fossils in breeding position in oviraptorids143 is striking (fig. 50). Norell et al. (2018) conclude that oviraptorids must have been distinctly social animals. Clark, Norell, and Chiappe (1999) report an oviraptorid fossil preserved in bird-like breeding position over a nest (see Norell et al. 1995, 775).
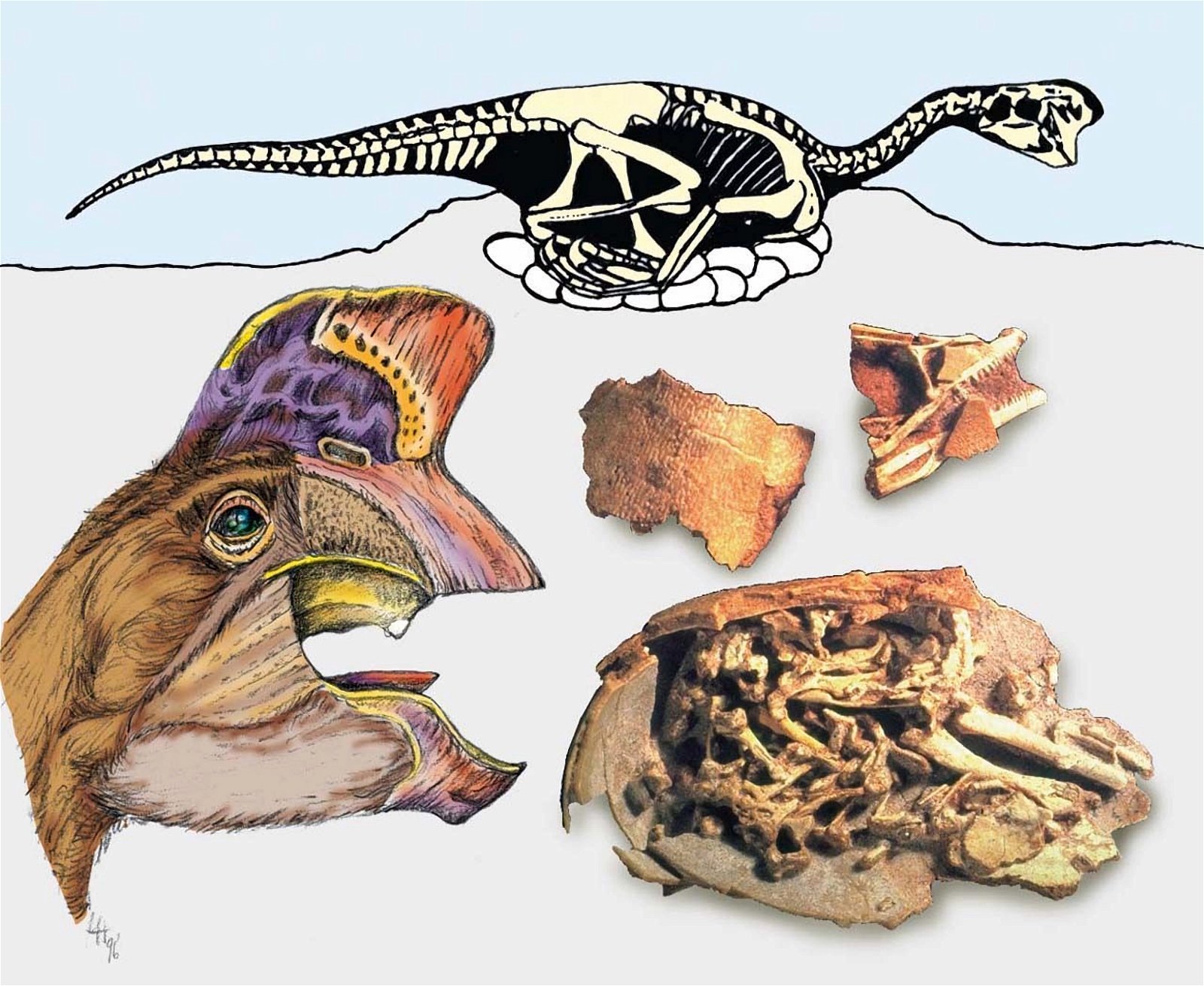
Fig. 50. Oviraptorid sitting on a nest (A; B head of an animal). The ensemble shows evidence of rapid burial. Embryos were fossilized even in the eggs (C) (A. after Norell et al. 1995; B. after Paul in Weishampel 1995; C. from Norell et al. 1996. Reproduced with permission from Science 266, © American Association for the Advancement of Science).
In rare cases, eggshell microstructure is preserved in theropod fossils, namely Troodon formosus and oviraptorids (Grellet-Tinner and Chiappe 2004, 199ff.). Varricchio and Jackson (2004b, 931) describe eggs of Troodon that show some avian characteristics.144 They conclude through their study that no eggshell feature clearly separates birds from theropod dinosaurs (Varricchio and Jackson 2004b, 935145, also Varricchio et al. 2013; 2018). The eggs of Troodon and other troodontids were said to be most similar to those of present-day birds in terms of their asymmetrical shape, low porosity, lack of ornamentation, and three-layered eggshell.146 Jackson, Horner, and Varricchio (2010) were able to confirm that the eggshells of Troodon were trilayered (see also Varricchio et al. 1997, 248).
The species of the oviraptorids and troodontids, in which brood care was proven, all originate from Upper Cretaceous strata (Zelenitsky and Therrien 2008; see fig. 51), the eggs of the dromaeosaurid Deinonychus from the Upper Lower Cretaceous (Cloverly Formation, Montana/USA; see Grellet-Tinner and Makovicky 2006). The fossil evidence thus emerges stratigraphically quite late. In contrast, dozens of genera of the Enantiornithes and Ornithurae are already known from the Lower Cretaceous.
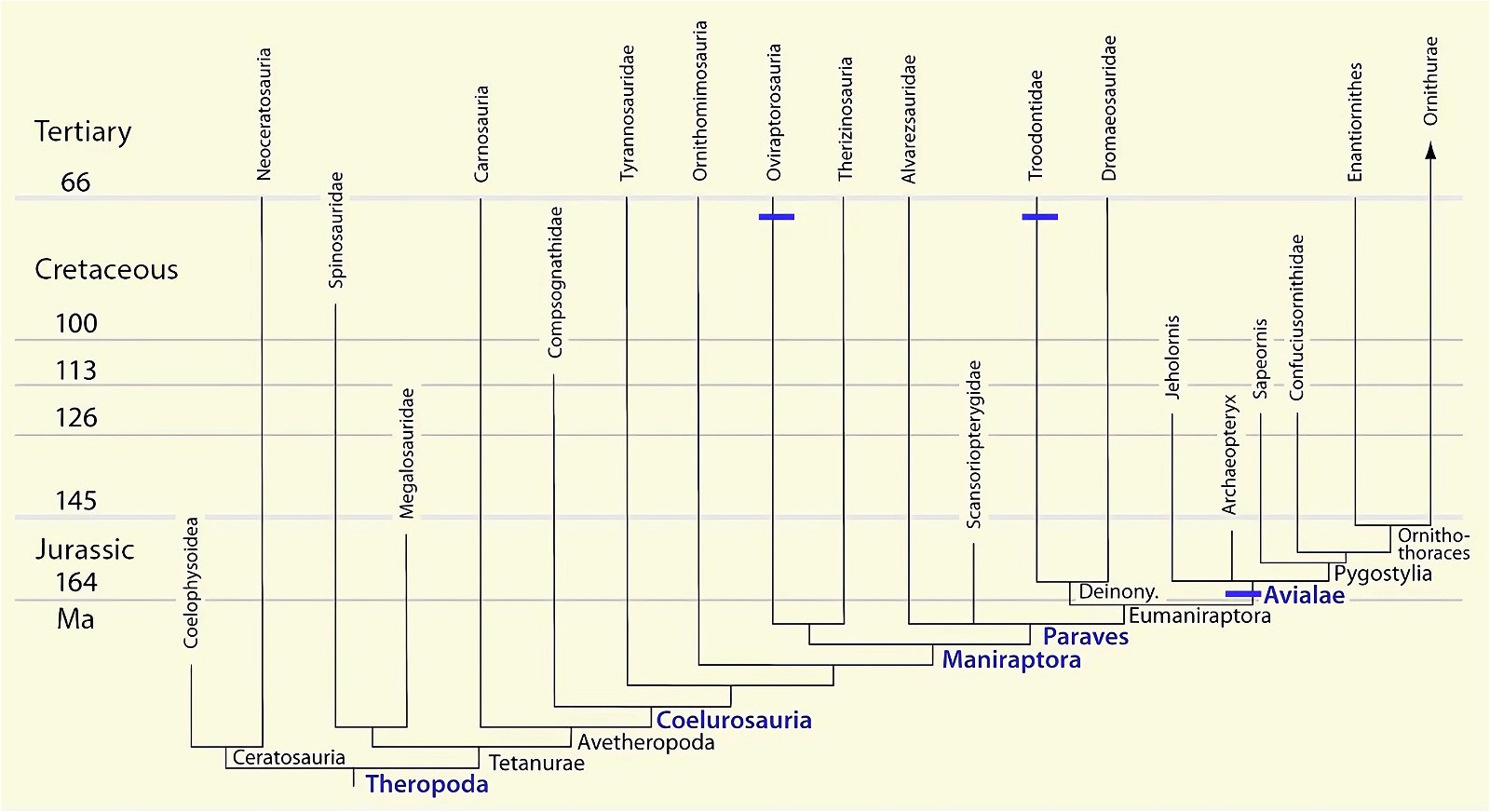
Fig. 51. Distributions of theropod and avian genera with avian-like characteristics in eggs and brood care (blue markings) (assembled from sources cited in the text).
Clutches and nests of Troodon formosus are interpreted by Varricchio et al. (1997, 248) as indicating that this species produced two eggs simultaneously (one per ovary) at an interval of one or more days (rather than en masse as in reptiles) and incubated the eggs by a combination of ground and body contact. This suggests the existence of two functional ovaries, the absence of egg turning, and partial egg burying, which are “primitive features” untypical of birds. On the other hand, there are features (as mentioned above) common with birds, namely, relative size of eggs and possibly their asymmetry, production of only one egg per oviduct per unit time (one day or more), an open nest, and brooding (see Varricchio and Jackson 2004a, 215; see Sato et al. 2005; Zelenitsky and Therrien 2008). The finding of paired eggs of Sinosauropteryx can also be interpreted as evidence for the existence of paired functional ovaries (Chen, Dong, and Zhen 1998).
In summary, according to Grellet-Tinner and Chiappe (2004, 208), the following characteristics of eggs and clutches connect birds with theropod dinosaurs: (at least) two layers of eggshells (bird eggs have three or more layers), aprismatic nature of the layers of eggshells, asymmetry of eggshells, brood care, and monoautochronic ovulation (eggs are laid in the interval of at least one day). While eggshell ornamentation is typical for dinosaurs, eggs in birds are usually smooth, which these authors interpret as reversion. However, even those theropod dinosaurs closest to birds lack some key reproductive features of present-day birds (Varricchio and Jackson (2016, 654, 661, 664), namely, the formation of only a single functional ovary, size of eggs, rotation of eggs in the nest147, and sediment-free nests.148 However, among birds, only Neornithes have sediment-free nests (Varricchio and Jackson 2016, 654, 675f.149). Finally, the reproductive biology of oviraptorids and troodontids differs markedly from that of most other theropod dinosaurs where something is known about them (Varricchio and Jackson 2016, 660).150
Wiemann, Yang, and Norell et al. (2018) published data on color pigments in fossil dinosaur eggs. Using high-resolution Raman spectroscopy, they examined eggshells from representatives of all major dinosaur groups and concluded that colored eggs (presumably for camouflage) represent a common heritage of theropod dinosaurs and did not arise independently multiple times. The type of pigment deposition in the shell structure was also consistent with that of present-day bird eggs.
Early Birds
In all fossil known birds, including the most basal such as Jeholornis, only one functional ovary was present. As far as is known, they were “modern” in this respect (O’Connor et al. 2014, 16151, see also Zheng et al. 2013). This, they argue, supports the hypothesis that the loss of an ovary is related to the weight savings required for flight. O’Connor and Zhou (2015) suggest this is a hallmark of birds.152 In contrast, they argue that there is evidence that follicle maturation was slow and thus similar to that in Crocodilia.
Even in the basal bird Eoconfuciusornis, only one cluster of follicles153 has been detected. This species, of all the species, shows the greatest known extent of hierarchy of follicles among the Cretaceous birds and is the most modern in this respect (Zheng et al. 2017).
Brood Care by Males
Varrichio et al. (2008) were able to demonstrate, based on the size of the nests and on anatomical features of the skeletons fossilized together with the nests, that brood care in Troodon, Oviraptor, and Citipati was performed by males. In birds, males and females perform brood care in over 90% of cases. Only in basal forms such as Palaeognathae154 do only males care for the nest, which fits the presumed phylogenetic sequence (see also Prum 2008). However, convergence cannot be excluded regarding this behavior, since there are many phylogenetic lineages between the oviraptorids and troodontids on the one hand and the Palaeognathae on the other hand (Prum 2008, 1800), about whose brood care nothing is known.
Brood Care in Ornithischians
Brood care is not only known in maniraptorans, which are close to birds, but has also been demonstrated in ornithischians, the large group of dinosaurs that is not in any close relationship to birds. In Psittacosaurus, for example, a larger group of juvenile forms was discovered with an adult form that appeared to have been rapidly embedded while still alive, which Meng et al. (2004, 145) interpret as evidence of brood care.
Already in 1979 Horner and Makela had described a find of 15 equally sized juvenile animals of the hadrosaur Maiasaura in nest-like arrangement and together with shell remains (Upper Cretaceous, Campanian). According to Horner and Makela (1979, 297), the fact that 15 juvenile individuals were fed and were together for a longer period of time speaks for distinct brood care. The authors mention three other examples of possible brood care in hadrosaurs and in a horned dinosaur (with fewer individuals in the presumed nest).155
Homology or Convergence?
Overall, little is known about egg structure, clutching, and brood care in dinosaur theropods, which are placed in an ancestral position to birds. Data about it are almost only available for some troodontids and oviraptorids from the Upper Cretaceous. Whether brood care was established early from an evolutionary point of view and can be considered original to all dinosaurs is therefore doubtful (Meng et al. 2004, 145156; see Zelenitsky and Therrien 2008, 812157). But also within the maniraptorans (see cladogram fig. 4) a homology is not assured. While the troodontids together with the dromaeosaurids are mostly classified as a sister group of the Avialae (which supports a homology), the oviraptorids stand further apart. Zelenitsky and Therrien (2008, 812) do present a cladistic analysis that features of eggs and reproduction are consistent with the topology of phylogenies based on skeletal features, but there are few finds to base this on (see also the cladogram of Varricchio and Jackson [2004b], for which the same is true158). Zelenitsky (2006, 209, 215) also states that in theropod dinosaurs there are also unique features in reproductive behavior for which there is otherwise no parallel in archosaurs.
Decisive for the decision homology or convergence would be knowledge about clutches and brood care in the intervening lineages (alvarezsaurids, scansoriopterygids) (see Grellet-Tinner and Chiappe 2004). Moreover, the assumption that not only Upper Cretaceous genera, but also Lower Cretaceous troodontids and oviraptorosaurs possess bird-like characteristics in clutching and brood care is obvious, but not self-evident. Stratigraphically, the theropod genera with proven (or very probable) brood care are fossil recorded much later than numerous bird genera.
Varricchio and Jackson (2004b, 935) note that their analysis of trait distributions reveals a striking degree of homoplasy (convergences or reversions) in the evolution of eggshell structure. For example, both the eggshells of Troodon and those of an unnamed egg type from their study show a three-layered structure as in many modern birds, but they lack the characteristic scale-like structure.159 In contrast, the eggshells of oviraptorids have only two layers but possess a scale-like structure. In turn, the eggshells of the theropod genus Lourinhanosaurus (unclear systematic position) have three layers but no scale-like structure, and the eggs are relatively small (Varricchio and Jackson 2004b, 935).
Conclusion
In oviraptorids and troodontids, a number of avian characteristics are pronounced in eggs, clutches, and brood care—only one egg per oviduct per day, large eggs relative to body size, complex eggshell structure, egg asymmetry, and brood care. Varricchio and Jackson (2004b, 936) conclude that most of the eggshell features considered typical of avian eggs have already evolved in theropod dinosaurs.160 However, their distribution in the system requires some degree of convergence. The extent of this cannot be estimated at this time due to the paucity of data with the associated uncertainty about trait expression in intermediate lineages of theropods.
Quotes on the Widespread Occurrence of Convergences
The following are some quotes from researchers expressing the very common occurrence of convergences (emphasis added in each case).
- “Homoplasy is common among theropod dinosaurs” (Xu and Pol 2013, 327). Case study: “Lee and Worthy (2012) found that the deinonychosaurian status of Archaeopteryx is supported by more synapomorphies but that these characters are more homoplastic than those supporting the avialan affinities of Archaeopteryx (which are fewer but less homoplastic).” (Xu and Pol 2013, 327)
- “An accurate phylogeny is the basis for understanding avian origins, but coelurosaurian systematics is plagued by large amounts of missing data and prevalent homoplasies, . . .” (Xu et al. 2009, 434)
- “Convergent evolution and mosaicism in character evolution among paravians is commonplace.” (Turner, Makovicky, and Norell 2012, 137)
- “This uncertainty is due to real observed homoplasies; suites of derived characters shared with other different clades of coelurosaurs whose distributions cannot be resolved without some reversals or convergences.” (Holtz 2001, 116)
- “Considering the distribution and combination of morphological characters in the fossil record it goes clear that many or even most characters considered typical of birds, like reduction of teeth, reduction of manual claws, the horny bill, the pygostyle, reduction of the fibula etc., evolved more than once.” (Peters 2002, 353)
- “the fact that avian features have arisen repeatedly and independently in theropod evolution now seems to be an inescapable conclusion.” (Witmer 2002, 5)
- “The distribution of ‘avian’ characters strongly suggests evolution in the maniraptoran clade was highly homoplastic.” (O’Connor and Sullivan 2014, 4, emphasis added)
General Conclusions from the Individual Studies
The individual studies have shown that the bird-typical features are distributed in the system of theropods and Cretaceous birds in such a way that convergences or reversions are assumed almost throughout, often several times. Evolutionarily, this means that these traits must have evolved independently two or more times. O’Connor, Chiappe, and Bell (2011, 40f) conclude that the distribution of avian-like features such as beak, furcula, sternal plates, uncinate processes of the ribs, retroverted pubis, distally nonfused pubic bones, parallel orientation of pubis and ischium, and the formation of a pygostyle are common within maniraptorans in a way “that makes it difficult to determine a clear pattern of origin of the features for most of them. This suggests an earlier origin of many of these traits, while their occurrence in apparently unrelated groups of theropods, and their absence in primitive birds, suggests a highly homoplastic evolutionary history.”161 Numerous authors note that there are confusing relationships and convergences are common (see the citations on convergences and on confusing relationships; see also tables 2 and 3).
Table 2. Brief overview of the results of the individual investigations with short summaries on the question to what extent the characteristics in question can be interpreted as characteristics in hypothetical bird ancestors
| Feathers | Flat and today's feathers comparably developed in some troodontids (Anchiornis) and dromaeosaurids (Microraptor), but different type of flight (four-winged) than in Avialae. Whether hair-like or simply feathered body appendages should be called feathers is debatable or theory-dependent (see Junker 2017). |
|---|---|
| Furcula | At the base of theropods, however, the homology with the furcula of birds is questioned by some researchers. |
| Beak | Not a precursor feature, but convergent in theropod dinosaurs. |
| Brain characteristics, EQ | Probably convergent in troodontids and oviraptorids, thus probably not an avian precursor trait. |
| Gastralia | Although present in some theropod dinosaurs, some trait expressions may have evolved convergently. |
| Uncinate processes of the ribs | Feature absent in basal birds, therefore likely convergent in theropod dinosaurs and thus probably not an avian precursor feature. |
| Sternum | Distribution of species with and without sternum does not indicate correlation with presumed phylogeny; probably convergent, thus probably not an avian ancestral trait. |
| Pneumatization of bones | Commonly present in theropod dinosaurs. |
| Flow-through respiration | Probably universally present in theropod dinosaurs. |
| Pelvic features | Convergent in theropod dinosaurs, probably not an avian precursor feature. |
| Pygostyle | Partly convergent in theropod dinosaurs; not an avian precursor feature. |
| Reduced fibula | Fibula not reduced in many Mesozoic birds. Reduction convergent in theropod dinosaurs. |
| Wrist | Bird-like mobility widespread in theropod dinosaurs, but homologies partly uncertain. |
| Eggshells | Bird-like expression common in theropod dinosaurs. |
| Only one functional ovary | Only in birds. |
| Brood care | Recorded in some theropod dinosaurs, but in forms much younger than a large proportion of Mesozoic birds, thus uncertain as a bird precursor trait. |
Table 3. Tabulation of the results assembled in table 2. It is striking that many of the avian features examined that are present (+) in theropod dinosaurs are not formed in those forms that are considered immediate avian precursors, but must have evolved convergently (yellow underlay). A large proportion of the other features examined are considered to be general theropod features (light red underlay) and are therefore also not well suited to support a gradual origin of avian features in theropod dinosaurs. +, present; –, absent, ? unclear.
| present at base of Avialae | convergent in theropods | general feature of theropods | present in theropods, homology uncertain | |||
|---|---|---|---|---|---|---|
| Pennaceous feathers | +? | -? | - | - | ||
| Furcula | + | -? | + | + | ||
| Beak | - | + | - | - | ||
| Brain features, EQ | -? | + | - | - | ||
| Gastralia | + | (+) | - | - | ||
| Uncinate processesof the ribs | - | + | - | - | ||
| Sternum | - | + | - | - | ||
| Pneumaticity | + | - | + | - | ||
| Flow-through respiration | + | - | + | - | ||
| Pelvic features | - | + | - | - | ||
| Pygostyle | - | + | - | - | ||
| Reduced fibula | - | + | - | - | ||
| Wrist | + | - | (+) | + | ||
| Bird-like eggshells | + | - | + | - | ||
| One functional ovary | - | - | - | - | ||
| Brood care | +? | +? | - | - | ||
In some cases one would have to assume under evolution-theoretical guidelines that “avian features in dinosaurs” are lost again after their emergence, before birds have evolved. In such cases, they can consequently not be precursor traits.
Survey Representative?
The above study may not be representative. The selected characteristics are incomplete. However, the selection was not deliberately selective, but was based on the available material. The result shown in fig. 1 and table 3 was not anticipated by this author. In this respect, it is quite surprising. The starting point of the investigations was the observation that many authors point out that the presumed transition area dinosaur–bird is characterized by very many convergences. The author has followed up this observation more exactly by the present investigation.
Quotes on Unclear Evolutionary Relationships
Below are some quotes from researchers expressing that relationships within theropod dinosaurs close to birds are unclear.
- “the increase in specimen data has complicated rather than clarified the problem of identifying the avian sister-group, revealing a mosaic of ‘avian’ morphologies inconsistently distributed among purportedly closely related clades of non-avian dinosaurs. . . . Basal birds themselves possess disparate morphologies and do not provide a clear picture of the plesiomorphic avian taxon: Archaeopteryx strongly resembles troodontids such as Anchiornis and Xiaotingia (Turner, Makovicky, and Norell 2012; Xu et al. 2011), while the robust skull of sapeornithiforms most strongly resembles those of recently discovered basal oviraptorosaurs such as Caudipteryx (Ji et al., 1998) and of the scansoriopterygid Epidexipteryx.” (O’Connor and Sullivan 2014, 4)
- “As a result of the high amount of homoplasy that characterizes derived maniraptoran evolution, the identity of the avian sister taxon remains debated despite the rapid accumulation of morphological data.” (O’Connor and Sullivan 2014, 23)
- “Each of these clades [Dromaeosauridae, Troodontidae, Deinonychosauria] possesses a different combination of avian characters distributed among the included taxa, . . .” (O’Connor, Chiappe, and Bell 2011, 45)
- “Inferred relationships between theropod clades are complex and have changed dramatically over the past thirty years with the emergence of cladistic techniques” (Hendrickx, Hartman, and Mateus 2015, 1). “Though one might expect few major changes in theropod relationships in the future, large portions of theropod phyletic history remain obscure; . . .” (Hendrickx, Hartman, and Mateus 2015, 34)
- “The discovery of numerous small-sized paravian theropods in the Late Jurassic and Early Cretaceous of China in the past decades have greatly enhanced our understanding of basal paravian anatomy and evolution. However, they also provided sometimes confusing evidence of widespread convergence and parallel evolution in this clade, . . .” (Rauhut, Foth, and Tischlinger 2018, 83).
Theropod Characteristics or Lack of Avian Characteristics in Basal Birds
On the one hand, many bird-typical features are present in theropod dinosaurs. Conversely, a number of features typical of modern birds are absent in many early birds, whether in individual genera or in entire groups. “The earliest birds lacked many key features related to powered flight in modern birds, and probably had primitive flight capabilities that varied substantially between groups” (Brusatte, O’Connor, and Jarvis 2015).162 Much has been discussed in the previous sections, which need only be recalled here. Due to the fame of the “Urvogel”, the absence of a pygostyle in favor of a long caudal spine, the absence of a beak, and absence of a bony sternum are familiar features of Archaeopteryx (Brusatte, O’Connor and Jarvis 2015; a gastral basket is also not developed). Many enantiornithine birds did not possess a sternal keel (Brusatte, O’Connor, and Jarvis 2015, 892). Jeholornis also possessed a long caudal spine instead of a pygostyle (see above). A toothed jaw without a beak was present in a number of Cretaceous bird genera.
More Examples
Benson and Choiniere (2013, 2) point out that the proportions of the hind limbs of most Mesozoic birds are similar to the proportions in theropod dinosaurs. The short-tailed genus Sapeornis possessed a shoulder girdle similar to that of deinonychosaurs (Xu et al. 2014, 2). The furcula of basal birds such as Archaeopteryx was not formed as an energy-storing elastic brace, but may have served as an attachment point for flight muscles (see above). Gastralia were only developed in Mesozoic birds. There are enormous differences in the construction of the pelvis between birds known from Cretaceous strata and present-day birds. In many Mesozoic birds, the fibula is not greatly reduced as in present-day birds. In the discussion of individual features, the unsystematic distribution of bird features in dinosaurs was pointed out. This situation applies accordingly to the distribution of bird-untypical features in Mesozoic birds.
Avian Precursors or Secondarily Flightlessness?
In the case of some genera or entire groups that are interpreted as bird precursors, a minority of researchers working in this field discuss whether they are secondarily flightless birds. In this case, their features would be eliminated as evidence for a gradual transition from dinosaurs to birds, and this would significantly change the overall picture. Some researchers make a strong case for this interpretation, especially in the case of oviraptorids (Maryañska, Osmólska, and Wolsan 2002). However, this interpretive possibility is also discussed in the dromaeosaurids (Czerkas and Feduccia 2014, 850), the scansoriopterygids (Chatterjee and Templin 2012; Czerkas and Feduccia 2014; Zhang et al. 2008), and the alvarezsaurids (Altangerel et al. 1993; Chiappe 2002b; Peters 2002, 349). For the alvarezsaurids, however, the interpretation as secondarily flightless birds has now become less likely due to new finds (Xu et al. 2018; see below). It has also been brought into play for Archaeopteryx by Michael Habib that its flight ability may have been partially forfeited (Kaplan 2013). He concludes this by comparing leg lengths and feather symmetries in Archaeopteryx and present-day birds. These are similar in Archaeopteryx as in present-day secondarily flightless birds.163
In the section on rib construction, Paul (2001, 479) was already quoted as saying that features of the respiratory apparatus, such as the uncinate processes on the ribs, were more derived in dromaeosaurids and oviraptorosaurids than in Archaeopteryx and were developed similarly to secondarily flightless birds, which could indicate a secondarily flightless status (see also Codd et al. 2008). Maryañska, Osmólska, and Wolsan (2002) argue for an “avian status” of Oviraptorosauria based on a cladistic analysis (compare fig. 52 with Fig. 4).164
Fig. 52. Cladogram after Maryañska, Osmólska, and Wolsan (2002), according to which the oviraptorosaurids are nested within the birds (Avialae).
The basal oviraptorosaur genus Caudipteryx also exhibits a number of bird-typical features, most notably flat, symmetrical feathers with a shaft (Martin and Czerkas 2000, 691165; Feduccia 1999b, 4742166; Wellnhofer 2002, 470, 474167). The construction of the hand is to be understood hardly differently than as inheritance of a flight-capable ancestor (Martin and Czerkas 2000, 691).168 According to Peters (2002, 350), Caudipteryx has anatomical features very similar to flightless ratites, which gives a clear indication that Caudipteryx is a bird and had flightless ancestors. Because of the smallness of the feathers and the forelimbs, Caudipteryx and also the in some respects similar Protarchaeopteryx may not have been capable of flight. However, it is implausible to consider these two genera as avian ancestors (Peters 2002, 349). The relative proportions of the posterior extremities of Caudipteryx are in sharp contrast to other bipedal dinosaurs. They are indistinguishable from ratites169 and the center of gravity is significantly more forward in the body than in all bipedal dinosaurs, according to Jones et al. (2000, 727). Therefore, the interpretation of Caudipteryx as a secondarily flightless bird is most plausible, even though cladistic analyses place the genus among the coelurosaurs.170 Ruben and Jones (2000, 594) consider that features that would clearly support an avian precursor status are either absent or questionable in Caudipteryx.171
Feduccia and Czerkas (2015, 1067) enumerate a whole series of bird-typical features in Caudipteryx172 and still particularly refer to the possession of an aerodynamic propatagium (flight skin in the area of the wings) in some specimens of Caudipteryx, which also argues that this genus and thus the oviraptorosaurs as a whole were derived flightless ground dwellers.173 Propatagia were also discovered in the dromaeosaurid Microraptor (Agnolín and Novas 2013), the troodontid Anchiornis (Wang et al. 2017b), and Scansoriopteryx (Czerkas and Feduccia 2014), which, among other indications, supports flight capability or possibly secondary flight loss in flightless genera of these families (see Feduccia and Czerkas 2015, 1070).
Kundrát (2007) considers it possible that the oviraptorid Conchoraptor, with bird-like brain features, descended from ancestors capable of flight.174
Usually, it is argued that the cladograms are less contradictory if the groups concerned are placed in an ancestral position to the birds. But the cladistic argument is not very strong, because it has been shown more and more that convergences can occur frequently. This, however, calls into question the parsimony principle that underlies the creation of cladograms. This would be even more true if some biologists were right, who meanwhile even claim that convergent development is something like the “way of life” of evolution.175 Zhou et al. (2000, 252), while considering Caudipteryx more likely to be an avian ancestor among theropod dinosaurs on the basis of cladistic results, note that the parsimony principle of cladism is “philosophically untestable”. So, it must be conceded that there is no compelling evidence that it was not a flightless bird.
Dyke and Norell (2005), on the basis of their analysis, counter that there is no reason, either in terms of phylogeny, morphometrics, or any other facts, to conclude that Caudipteryx is anything other than a small theropod dinosaur. Chiappe and Dyke (2002, 109) consider an avian status of Caudipteryx implausible because problematic reversion would have to be assumed such as reevolution of a bony tail,176 development of new phalanges and renewed elongation of fingers, changes of the sternum back to small and separate sternal plates, and reversion of fusion of multiple bones and pelvic features.177Throughout, the arguments for avian status were based on faulty methodology in their opinion (Chiappe and Dyke 2002, 107).178 For Witmer (2002, 11), the primitive features of Caudipteryx also argue against an avian status for this genus. Consensus has not been reached on the status of Caudipteryx and the Oviraptorosauria as a whole.
Noteworthy in this context is the fact that flightlessness is always considered secondary in present-day species with true feathers and is very common (Chiappe 2002a).179 Today’s flightless birds live on sites (mainly islands) where flight ability could be forfeited without loss of fitness. A path back to flight ability was never taken and it is therefore even less likely for first-time flight acquisition.
Striking Jumps
A gradual appearance of various individual traits (see preliminary remarks) does not automatically mean that the emergence of these traits is also plausible in evolutionary theory. This was explained above with some examples. On the other side, some traits appear relatively abruptly. For example, Xu et al. (2014, 1341) write: “However, new data also highlight occasional bursts of morphological novelty at certain stages particularly close to the origin of birds and an unavoidable complex, mosaic evolutionary distribution of major bird characteristics on the theropod tree.” And Brusatte (2017b, 55) notes that the first birds evolved at exaggerated rates, namely, once the building blocks (“lego-kit”) were complete, incredible evolutionary potential was unlocked, so to speak.180
A relatively sudden appearance has already been noted for some traits above. This is especially true for the pygostyle (for example, Rashid et al. 2014; Wang and Zhou 2017, 6). Further, the avian beak emerges suddenly in fully developed form with Confuciusornis. Other genera classified as basal, Archaeorhynchus and Hangshanornis, also have a beak and are toothless or nearly so. Although there are many genera with diverse expressions of dentate jaws that also have a beak (see above), stratigraphically the first forms with fully developed beaks appear abruptly.
In the section above on clutches and brood care, it was mentioned that all fossil known birds, including the most basal such as Jeholornis, had only one functional ovary as far as is known, and even the geologically oldest birds were “modern” in this respect (O’Connor et al. 2014, 16, see also Zheng et al. 2013).
Dececchi and Larsson (2013, 2741) have shown that when body size was taken into account, there was no trend of an increase in forelimb length in theropod dinosaurs and this suddenly changed in birds.181 Similarly, Carrano (2000, 489) notes with respect to pelvic girdle and hind-limb morphology that this has been remarkably uniform in theropod dinosaurs, showing little variation toward specialized forms. This apparent evolutionary stability makes the transition of the locomotor system to birds even more remarkable.182
According to Sanz et al. (2002), the formation of the derived wing proportions and the rod-shaped raven leg correlates with the shortening of the tail and the formation of a pygostyle in early birds. Accordingly, the decoupling of the hind leg and tail and the new connection of the thoracic and tail modules to form the flight apparatus occurred simultaneously.183
Zheng et al. (2014a, 7) point out that the alimentary canal was already formed in basal ornithuromorphs very similar to present-day birds.184
The architectures of the shoulder girdle of Archaeopteryx, Jeholornis, and Sapeornis on the one hand and the advanced Ornithothoraces on the other hand are clearly different. In the former, the supracoracoideus muscle was probably attached only to the coracoid bone, whereas in the latter a supracoracoideus traction was developed and the muscle also attached to the sternum. The two constellations probably reflect different features of locomotion (Mayr 2017b, 865). Sanz et al. (1996, 442) see a structural gap between Archaeopteryx and the basal Ornithothoraces that would have significantly enhanced their flight capabilities.185
New examination methods such as laser-assisted fluorescence microscopy have recently made it possible to detect previously unknown details of soft tissue anatomy (ligaments, muscles, tendons). This has shown that Confuciusornis (fig. 53) possessed “a suite of relatively modern soft tissue structures that are more advanced than may be expected” (Falk et al. 2016, 6). In addition, well-developed and resistant flight skins (propatagium and postpatagium on the inner and outer sides of the wings) were detected, which, according to Falk et al. must have enabled enormous lift and, together with the robust feather shafts, argued for the ability to fly actively. Using evolutionary theory, the authors conclude that the flight skin system evolved early and may be a common feature (synapomorphy) of all birds (Falk et al. 2016, 8).186 Based on the new findings, the researchers conclude that Confuciusornis could fly short distances well and possessed many relatively “advanced” avian anatomical features. Overall, then, the Confucius bird was by no means primitive, which is why the authors speculate, in terms of evolutionary theory, that its modern features must have arisen much earlier than previously thought. Older rock strata could perhaps provide relevant answers about precursors.187 Soft-tissue features were also identified in an unnamed mating bird from Lower Cretaceous strata, characterized as surprisingly modern and suggestive of very good flight ability (Navalón et al. 2015; fig. 54), so a revision regarding flight ability was made in this case as well. The enantiornithine birds, to which the find is included, are now considered good flyers. The bastard wing was also already developed among geologically ancient forms of the opposite birds (Zhou 2004, 457).188

Fig. 53. Numerous skeletons of Confuciusornis sanctus are fossil preserved. Eduard Solà, “Confuciusornis sanctus fossil specimens exhibited in Cosmocaixa, Barcelona,” https://en.wikipedia.org/wiki/File:Confuciusornis_sanctus.jpg, CC BY-SA 3.0.
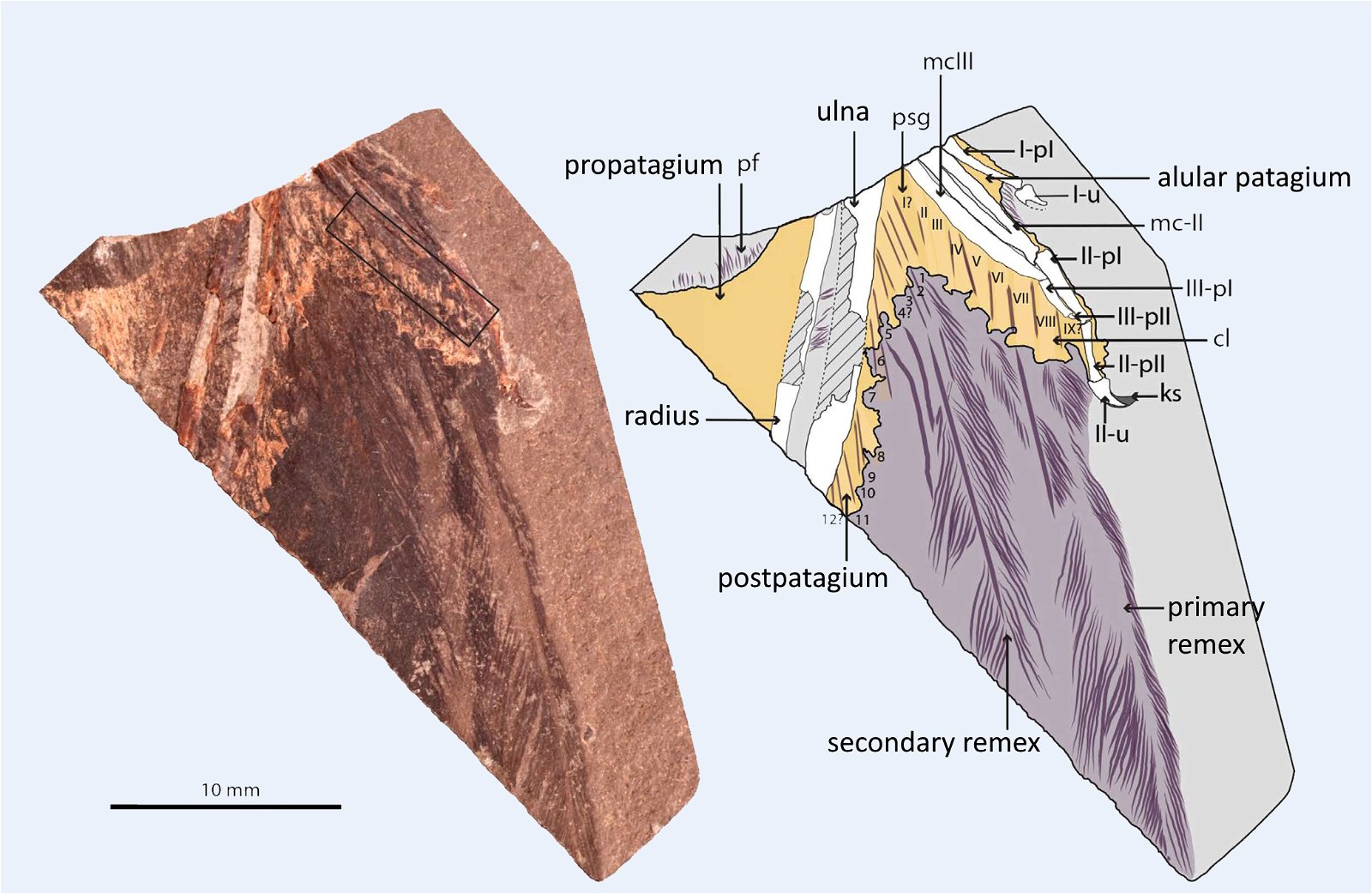
Fig. 54. Fossil and interpretive drawing of enantiornithine MCCMLH31444 from Las Hoyas, Spain. The framed portion includes the region of transition between bone and soft tissue. Abbreviations: I, II, III refers to fingers I, II, and III, cl calamus; ks keratinized sheath, mc metacarpal, p phalanges, pf down feathers, u claw (from Navalón et al. 2015, modified), CC BY 4.0.
The Upper Jurassic troodontid Anchiornis, which is feathered with pennaceous feathers and is (incorrectly?) not considered a bird, was also found to have a flight skin using the new study methods (Wang et al. 2017b; fig. 55). The forearm, hand, lower leg, and foot each had 10–13 long flight feathers. Therefore, this four-winged form was probably able to fly much better than previously thought. The flight skin at the elbow helps modern birds take off from the ground. Thus, it may have helped Anchiornis achieve this ability as well, according to the scientists. However, this cannot be determined with certainty. Furthermore, the symmetry of the feathers clearly argues against the ability for active flight. The characteristics of Anchiornis contradict altogether in any case a gradual emergence of the flight ability.189 The distinct avian features only make sense if Anchiornis was capable of flight in some—so far probably unknown—way.

Fig. 55. Wings of Anchiornis under laser-induced fluorescence. The skin folds (patagia) in front of the elbow and behind the wrist were covered with feathers as in modern birds. Photo: Wang X. L., Pittman M. et al., Nature Communications 2017, CC BY-SA 4.0.
One of the geologically oldest enantiornithine genera, Protopteryx, classified as primitive, possessed an alula (Zhang and Zhou 2000). Thus, it must be assumed that the associated flight abilities occur early in the avifauna, presumably in the base of Ornithothoraces (see Zheng et al. 2017, 448190). This is because the genus Archaeorhynchus, placed at the base of Ornithothoraces, also possessed an alula (Zhou and Zhang 2006b). In contrast, an alula has not been recorded in the genera Archaeopteryx, Confuciusornis, Jeholornis, and Sapeornis, which are considered basal (Peters and Ji 1999; Zhou and Zhang 2003a, b, 2006a).
All of these findings suggest that flight capability has emerged abruptly, as it were, as a total package.191 Given the numerous requirements for active flight, this is ultimately not surprising.
Soft tissue is fossil preserved from the basal ornithuromorph Archaeorhynchus, and it can be concluded from the fossil remains that the lungs were “very similar to the lungs of present-day birds” (Wang et al. 2018). These authors see this finding in a set of features on soft tissue systems (for example, digestive and respiratory systems) that are typical of birds living today and were established early, before changes in the skeleton occurred.192
Conclusion
Many avian traits are classified as early established under evolutionary theory and abruptly emerge in fossils. This situation is a challenge for evolutionary mechanisms, as rapid emergence is not to be expected under evolutionary theory.
Early Diversity and Mosaics
On the basis of the distributions of traits in early birds and the dinosaurs close to them and their stratigraphic positions, on the one hand, it is a picture of different mosaics and consequently of a network of similarity relations; on the other hand, a rather abrupt appearance of multiple forms emerges. Both do not correspond to earlier formulated evolution-theoretical expectations. In addition, the following are some examples of the networked situation and the fast-appearing variety.
Cross-linked Relationships
Some researchers note that trait relationships among taxa tend to be reticulate rather than tree-like.
- Wellnhofer (2000, 37) assesses the situation based on the discovery of Confuciusornis as follows: “With this new finding, the phylogeny of birds seems to have proceeded in an increasingly complex manner, in bushy branching and with parallel developments rather than in a straightforward sequence with Archaeopteryx as the central ancestral form.”
- “[T]he original diversification of birds was probably also a complicated bush with many extinct lines that may at one time have been more advanced in some features than their ultimately more successful contemporaries” (Hou et al. 1999, 681). Confuciusornis must be regarded as an early branch of a bush-like radiation that was neither a precursor of modern birds nor of opposite birds.193
- The best known witness of this situation is the famous urvogel Archaeopteryx, because despite possessing “modern” feathers, it is placed by many researchers on a side branch that does not lead to other birds due to special traits (Hou 2001, 7194; Martin 1985, 182195; Shipman 1998, 116196; Zhou and Hou 2002, 179197; Xu and Pol 2013, 331198).
- Sumida and Brochu (2000, 492) state that avian features already present in dinosaurs were distributed among different species and that no species possessed all of these characteristics.199
- “As has been the case with the enantiornithines, the increase in ornithuromorph taxonomic diversity has not resulted in greater systematic clarity—rather the known diversity shows no clear pattern of character acquisition.” (O’Connor and Zelenkov 2013, 1280)
- Xu et al. (2014, 1341) write that the main avian features show a complex mosaic evolutionary distribution on the theropod tree.200
Fast Diversity
Some researchers note that, assuming evolutionary origination, rapid changes must be assumed (which would be implausible in evolutionary theory).
- “This discovery, together with many others in recent years, suggests that by the Early Cretaceous, early birds had not only diverged significantly in morphology, size and ecology, but had also differentiated with respect to feeding adaptation.” (Zhou and Zhang 2002, 409).
- “There is now a picture of rapid radiation of birds after the initial establishment of flight with at least three significant lineages—Confuciusornithidae, Enantiornithes and Ornithurae. . . . Each shows a considerable degree of specialization, with apparently competent flight performance broadly comparable to that of extant species, albeit with less overall morphological diversity.” (Rayner 2001, 372)
- Sullivan, Xu, and O’Connor (2017, 3) speak of “rapid diversification of aerodynamic structures” in the Paraves, of great diversity of bauplans with respect to skeletal and integumentary structures in Yanliao (Upper Jurassic) and Jehol (Lower Cretaceous), there is a “high level” of “experimentation” (for this term, see below), and homoplasies and exaptations with respect to the aerodynamic apparatus.
For example, the Lower Cretaceous enantiornithine Longipteryx (fig. 56) had relatively long and powerful wings and a very well-developed flight apparatus, and probably specialized in fish feeding as a predator lurking on shores (Zhang et al. 2001). Zhou (2004, 457) evaluates this as evidence that ecological diversity increased rapidly in the Lower Cretaceous.201

Fig. 56. Longipteryx, Beijing Museum of Natural History. Jonathan Chen, “Fossil specimen of Longipteryx chaoyangensis on display at the Beijing Museum of Natural History,” https://en.m.wikipedia.org/wiki/File:Longipteryx-Beijing_Museum_of_Natural_History.jpg, CC BY-SA 4.0.
The enantiornithine genus Huoshanornis, also from the Lower Cretaceous, possessed a relatively “modern” hand that allowed “extraordinary maneuverability,” which, according to Wang et al. (2010, 436), “highlights once again the rapid evolution the avian manus.”202
O’Connor et al. (2015b, 1559) note that a remarkable range of complex forms of the sternum has rapidly evolved within birds.
Dececchi and Larsson (2013) showed, on the one hand, that the relative elongation of the forelimb in putative avian progenitors is an allometric effect of the reduction in body size, yet when body size is taken into account, there is no trend of forelimb elongation. On the other hand, there are sudden significant differences in the relation of the forelimb and hindlimb skeleton in birds compared to theropod dinosaurs, namely, there would be a rapid decoupling of forelimb and hindlimb in relation to body size at the origin of birds.203 Early birds would also have rapidly diversified ecologically.204
- Brusatte, O’Connor, and Jarvis (2015, 893) note that there has been a “huge spike” in the rate of anatomical evolution in the earliest birds.205
- “The refinement of flight capability and manoeuvrability and the evolution of a fully opposable digit for perching proceeded rapidly once primitive avians were airborne.” (Sereno 1999, 2143)
- “Frond-tailed birds, ribbon-tailed birds and fan-tailed birds all co-existed during the rapid diversification of avialans in the early Cretaceous.” (Martyniuk 2012, 28).
Of all species, the oldest known genus of ornithurans, the blackbird-sized Archaeornithura from northern China (fig. 57), belongs to the derived forms of this group (Balter 2015, Wang, Zheng, O’Connor et al. 2015). Wang, Zheng, O’Connor et al. (2015, 6) speak of inconsistencies between stratigraphy and phylogeny. This, they argue, necessitates the assumption of so-called ghost lineages, that is, the existence of evolutionary lineages for which fossil evidence is lacking. The whole group of ornithurans does not appear in the fossil succession in the form of a growing diversity, but rather abruptly in differently differentiated forms (see also O’Connor and Zelenkov 2013, 1280).

Fig. 57. Reconstruction of Archaeornithura, one of the geologically oldest genera of Ornithurae. Credit: Zongda Zhang/Institute of Vertebrate Paleontology and Paleoanthropology, Beijing.
- Also, the second major group fossilized in the Lower Cretaceous, the opposite birds (Enantiornithes), appears suddenly and in great diversity from the beginning of the fossil record and, moreover, simultaneously with the ornithurans (see Feduccia 2001, 142206). There are indeed genera that cannot be clearly assigned to the Enantiornithes or to the Ornithuromorpha (O’Connor and Zhou 2013). However, this is not primarily due to primitive traits, but to mosaic distribution of derived traits as well. For example, the ornithouran Schizooura lii has a Y-shaped furcula, a coracoid without lateral process, and a flat scapula, which is typical of enantiornithines, but on the other hand derived features such as an elongate sternum with a pronounced sternal keel, which in turn is lacking in the basal ornithouran Archaeorhynchus, whose sternum is more reminiscent of opposite birds (O’Connor and Zhou 2013, 903f; Zhou, Zhou, and O’Connor 2012).
The species Cruralispennia multidonta, which is placed among the opposite birds, also possesses characteristics typical of the other large Cretaceous bird group, the Ornithurae (“bird-tails”). Cruralispennia occupies a derived position among the opposite birds and is not interpretable as a transitional form between the two groups. Moreover, this genus is among the oldest birds after Archaeopteryx—a “stratigraphic-phylogenetic discrepancy” (Wang et al. 2017d).
- “Early Cretaceous birds show a wide range of specializations of the wing and shoulder girdle bones.” (Mayr 2017b, 860)
Contradictory Distribution of Characteristics
The situation of a “complicated bush” stems from the fact that combinations of features of different genera are contradictory, so that their graphical representation is more easily reticulate than tree-like. Here are some examples:
- Zhongornis haoae, a basal bird was toothless (advanced feature) but possessed similarities to basal oviraptorosaurs (for example, a short, deep skull) and to scansoriopterygids (hand and pelvic features) (O’Connor and Sullivan 2014).
- The arboreal opposite bird, Liaoningornis, had a toothed jaw, while the ground runner, Confuciusornis, found in the same strata and considered more primitive, had a horned beak. In contrast, adaptations to flight were again more “modern” in Liaoningornis than in Confuciusornis. The sternum is well developed and has a sternal keel as an attachment point for the flight muscles. Together with a wide and reinforced thorax, this suggests that Liaoningornis possessed air sacs and a well-developed respiratory system like modern birds (Hou et al. 1996).
- The “primitive” bird genus Jeholornis had altogether a more modern anatomy compared to Archaeopteryx and in contrast to the latter’s pronounced dentition only three very small teeth in the lower jaw. On the other hand, with 27 vertebrae, it possessed a longer caudal spine than Archaeopteryx, which had only 23 caudal vertebrae, and the tail feathers are shaped more like those of dromaeosaurs than those of Archaeopteryx (O’Connor et al. 2012, 29207; Stokstad 2002; Zhou and Zhang 2002; Zhou and Zhang 2003a, 220). In addition, Jeholornis possessed special features, namely a unique fan-shaped strand of tail feathers, presumably for lift and as a stabilizer (O’Connor et al. 2013). According to these authors, this shows that tail development was complex and did not proceed as a simple transition from a pinnate to a fan tail.
- The temporal region of the skull of Confuciusornis is diapsid in construction, that is, with two completely separate temporal windows, which is considered a more primitive condition, whereas the otherwise more primitive Archaeopteryx possessed a more modern cranial anatomy (Peters 2002, 352). Martin et al. (1998, 288f.) note that Confuciusornis is a “curious evolutionary mosaic.” The anterior part of the skull is more advanced than that of Archaeopteryx and Cathayornis, while the posterior part is less advanced, they say. However, based on a recent study of 13 Confuciusornis skulls and a comparison with Eoconfuciusornis, which is dated 6 million years older, Elzanowksi, Peters, and Mayr (2018) conclude that the diapside construction is secondary and an autapomorphy. The shoulder girdle and hand are more similar to the expressions in Archaeopteryx than in other birds, whereas the pleurocoels on the vertebrae and the formation of a pygostyle are modifications indicative of the enantiornithines. Therefore, Confuciusornis as an outgroup is further evidence that there are many extinct lineages among early birds that (in evolutionary perspective) independently develop evolutionary innovations that became standard in later birds.
- The genus Cathayornis from the opposite bird group possessed a primitive skull similar to Archaeopteryx in Cathayornis after the inferred postcranial differences between the opposite birds and the ornithurans had already become established. According to Martin and Zhou (1997), this suggests that the modern ornithuran skull with avian-like mobility would have evolved independently in the ornithurans.
- Among the basal ornithurans, on the one hand, there are several toothless taxa (Archaeorhynchus, possibly the Hongshanornithids, Zhongjianornis), while the derived ornithurine genera Ichthyornis and Hesperornis possessed teeth (O’Connor and Zhou 2013, 904).
- According to Holtz (2001), the anatomy of the skull and metatarsus of troodontids support an assignment to the Arctometatarsalia (clade that included all Coelurosauria except the closer bird relatives, later combined into Ornithomimosauria), while the extremities and tail region suggest an assignment to the maniraptorans.208
- The ornithurian genus Chaoyangia possesses plesiomorphic (“primitive”) features in the pelvic region (unfused pelvic elements and a pubic symphysis) on the one hand, and large ossified hooked processes on the ribs (Chiappe 1995, 351), a “modern” shoulder girdle, and a well-developed sternum on the other (Zhou and Hou 2002, 176). The dentition, in turn, is a primitive feature (Martin and Czerkas 2000, 693).
- The oviraptorid Conchoraptor has a shortened olfactory tract and a cerebellum that covers the hemispheres of the brain, which is similar to the situation in modern birds, and the estimated ratio of brain mass to body mass is also within the range of modern birds. Most endoneurocranial features are less avian than corresponding structures in Archaeopteryx (Kundrát 2007, 499).
- Eoconfuciusornis is classified as a basal bird on the one hand, but on the other hand this species shows the greatest known extent of hierarchy of follicles209 among Cretaceous birds and is the most modern in this respect (Zheng et al. 2017).
- Zhang et al. (2008, 1107) refer to the appearance of scansoriopterygids as “bizarre” because their features are exquisitely combined in a mosaic fashion, some bird-like, some similar to those of oviraptorosaurs and, to a lesser extent, therizinosaurs. While some pelvic features are not otherwise known in theropods. This situation suggests that the morphological diversity among maniraptorans at the base of the origin of birds is greater than previously thought.210
Jianianhualong and Sinusonasus
Xu et al. (2017, 9f.) describe conflicting trait mosaics in the troodontid genera Jianianhualong and Sinusonasus:
Comparison of the morphological features of Jianianhualong with those of other troodontids shows that Jianianhualong has forelimbs and a pelvis very similar to those of basal troodontids, but a skull and hind legs more similar to those of derived troodontids. For example, Jianianhualong has forelimb and pelvis features found in basal troodontids such as Anchiornisand Sinovenator. . . . Many features of the skull and hind legs of Jianianhualong resemble those of derived troodontids . . .
In contrast, the situation is partially reversed for Sinusonasus: “The only other troodontid from Jehol that exhibits a mosaic of plesiomorphic and apomorphic osteological characters is Sinusonasus magnodens, which also has transitional anatomical features. Sinusonasus has a skull that closely resembles basal and non-derived troodontids, and a pelvis and hind legs that more closely resemble those of derived troodontids than basal ones.” Primitive and derived parts are thus here formed exactly the other way around, thus presenting conflicting traits.
They further state, “Troodontidae is a small theropod family displaying a relatively low morphological disparity, but the distinct spatial organization of the mosaic of plesiomorphic and apomorphic osteological features in Jianianhualong and Sinusonasus raises the possibility of modular evolution in troodontids. The mosaic of plesiomorphic and apomorphic osteological features identified in Jianianhualong and Sinusonasus appears to show some correspondence to the expression domains of Hox genes . . .”
General Assessments
- According to O’Connor and Zhou (2015, 333f.), the available data do not allow us to elucidate a pattern of acquisition of the derived avian skeletal traits.211
- “There is still mixed debate about the closest kin group from which the primordial birds emerged, although the fossil record has increased considerably.” (Kämpfe 2003, 40)
The diversity of early birds also includes relatively large forms, which is not unproblematic in evolutionary theory. For energetic reasons, an evolutionary transition should most likely occur with forms that are as small as possible (Zhang et al. 2014, 823). In this regard, the putative four-winged dromaeosaurid Microraptor fits the bill, but this genus is placed on a side branch because of its unusual flight apparatus (four-wingedness). In contrast, the feathered Upper Jurassic genus Anchiornis was relatively large, with a body length of a good half meter. The pigeon-sized Archaeopteryx and the approximately 30-cm Confuciusornis and Sapeornis, all considered among the most “primitive” birds, were also significantly larger than many Lower Cretaceous enantiornithines. Among these, however, there were also large forms (Zhang et al. 2014, 823).
Matching Precursors? Mismatched Mosaics
The statement, that bird characters evolved step by step already in dinosaurs, is relativized—besides the already discussed findings—also by the existence of numerous mosaic forms, whose combinations of characteristics do not fit into a transitional position and whose systematic position is for this reason often unclear or disputed. Some examples are discussed below.
Rahonavis
Rahonavis (fig. 58) was found in Upper Cretaceous sediments of Madagascar (Maastrichtian). Preserved are parts of the vertebral column and pelvis, legs, fore-limbs, and shoulder girdle. The genus combines a mosaic of theropod-like features typical of dromaeosaurids and avian-like features (Forster et al. 1998, 1915). Dromaeosaurid features include a long caudal spine of 13 elongated vertebrae, vertebral construction, a foot with unfused metatarsals, and a distinct sickle claw. The pelvic girdle shows similarities to Archaeopteryx, but also to Confuciusornis and enantiornithines (for example, fused sacral vertebrae). Clearly avian, however, are a reduced fibula, and knobs on the ulna (attachment points for arm wings). The glenoid fossa at the glenohumeral joint shows a bird-like lateral alignment (Chiappe and Dyke 2006; Forster et al. 1998, Wellnhofer 2009, 181). Also bird-like are the backward-facing first toe (for grasping around a branch) and the pneumatized bones. Possibly Rahonavis was an active flyer.
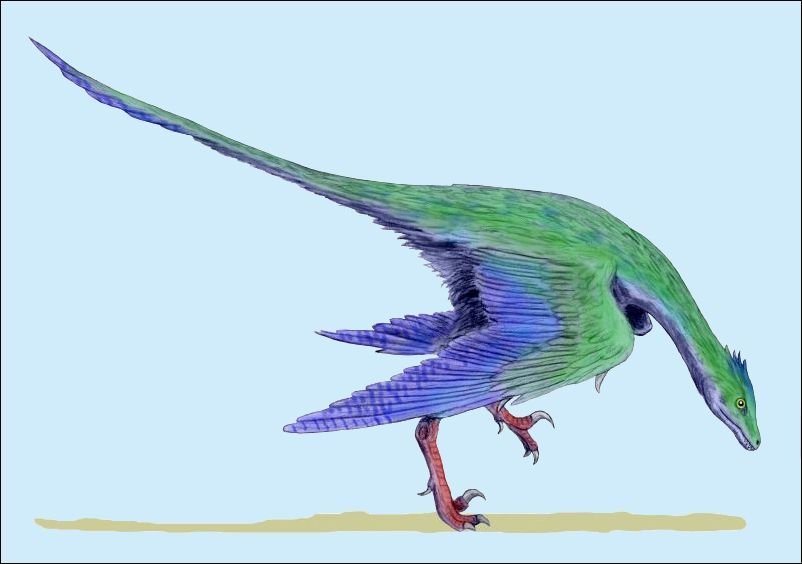
Fig. 58. Reconstruction of Rahonavis. Nobu Tamura, “Rahonavis ostromi, a dromaeosaur from the Late Cretaceous of Madagascar, pencil drawing,” https://commons.wikimedia.org/wiki/File:Rahonavis_BW.jpg, CC BY 2.5.
The systematic position of Rahonavis was discussed controversially. According to a cladistic analysis by Forster (1998), Rahonavis stands between Archaeopteryx and the Pygostylia and is to be included within the birds, so also more recently according to Agnolin and Novas (2013212). However, due to the dromaeosaurid features, the genus is now usually placed in the Unenlagiinae subfamily of dromaeosaurids despite the avian features (Makovicky, Apesteguía, and Agnolín 2005; Norell et al. 2006). Complicating matters further, the genus is dated about 80 million years younger than Archaeopteryx. Rahonavis is on the one hand more “primitive” than Archaeopteryx with respect to some features, but with respect to others it is clearly more avian, thus not suitable as a transitional form. Assignment to the dromaeosaurids would have as a consequence the avian features in Rahonavis having evolved convergently independently of the birds (Makovicky, Apesteguía, and Agnolín 2005, 1009).213 Overall, a place in the avian phylogenetic tree for this mosaic form with very primitive and highly derived features can only be found by putting it on a blind-ending side branch and assuming substantial convergence.
Mononykus
The genus Mononykus (fig. 59) is placed in the alvarezsaurids, a family of slender and long-legged animals that grew to half a meter to two meters in length. The systematic classification of the alvarezsaurids is highly controversial (Chiappe, Norell, and Clark 2002). This family can only be placed in the theropod and bird system assuming homoplasies (Novas and Pol 2002, 124).214 Cladistic studies initially placed this group closer to modern birds than to Archaeopteryx (Chiappe and Dyke 2002, 99; Chiappe, Norell, and Clark 1998, 277). According to Choiniere et al. (2014, 1f), the derived forms of Alvarezsauroidea possess remarkably avian-like features including a lightly built, mobile skull, several vertebral modifications, a keeled sternum, a fused carpometacarpus, a retroverted pubis and ischium, and delicate hind legs. Later analyses yielded results that argued against a closer relationship with birds and favored different positions for this group within the coelurosaurs (Chiappe and Dyke 2002, 99), implying that many bird-like features of the Alvarezsauridae should have evolved convergently in this group.

Fig. 59. Reconstruction of the skeleton of Mononykus. Thomas Cowart, “Mononykus skeleton, AMNH,” https://en.wikipedia.org/wiki/File:Mononykus_skeleton_fix.jpg, CC BY 2.0.
The genus Mononykus is taken as an example for the problems with the systematic position of the alvarezsaurids. Mononykus is a bipedal runner about 1 m long with a long neck and tail and long and delicate hind legs. Upper and lower legs are very similar to those of modern birds. However, metatarsalia 2–4 are not fused and metatarsale 3 is greatly shortened from the proximal end (Peters 1994, 412). Mononykus exhibits a distinct mosaic of characters. Very unusual is the short, robust anterior extremity with a single strong claw. Together with the keeled sternum, this could suggest a burrowing function of the forelimbs, especially since the highly modified forelimb resembles that of burrowing animals, while a sternal keel is a good attachment point for the powerful muscles required for burrowing (Zhou 1995). However, Chiappe, Norell, and Clark (2002, 117) believe that the body is too gracile and the forelimbs are too short for a burrowing lifestyle. Senter (2005), after a detailed study of the forelimbs, concludes that the lifestyle of Mononykus could not have been digging, but rather that the claw served to break open insect nests as in anteaters.
In addition to the formation of a sternal keel, a reduced fibula, fused carpal bones, and other features place Mononykus closer to birds than to Archaeopteryx, but other bird-typical features are absent (Altangerel et al. 1993, 623, 625; Padian and Chiappe 1998b, 23). On the other hand, Mononykus possessed a number of features, such as the sickle claw on the feet that are typical of dromaeosaurs (Chiappe and Dyke 2002, 102; Zhou 1995, 961).
Controversial Systematic Position
Similar to Rahonavis, avian status is also discussed for Mononykus due to its many avian-typical features (for example, Chiappe, Norell, and Clark 2002; Padian and Chiappe 1998b, 22; see Makovicky and Zanno 2011, 16), but this is rejected by some researchers. For example, according to Sereno (1997, 461), the absence of many avian characteristics seriously calls into question the avian status. If Mononykus is granted avian status, it was a sister genus to all birds except Archaeopteryx. Some features of alvarezsaurids are more similar to present-day birds than Confuciusornis (Makovicky and Zanno 2011, 16).215 However, an avian status would mean that we must assume a secondary flight loss in Mononykus. Problematic in this case would be the consequence that wings were transformed into burrowing organs (or, according to Senter [2005], for breaking open insect nests). This seems very doubtful.216
If, on the other hand, Mononykus is interpreted as the primary flightless sister group of all birds except Archaeopteryx, this would mean that the flight ability of Archaeopteryx evolved independently of the other birds, hence bird flight evolved twice independently (Norell, Chiappe, and Clark 1993), also a problematic consequence, since multiple independent origins of bird flight have usually been ruled out due to the enormous requirements for flight ability.
Finally, if Mononykus is classified outside of birds (as, for example, Zhou 2004), some bird-typical traits that were previously considered synapomorphies (homologies) must be classified as independently evolved, that is, convergent (Novas and Pol 2002, 122).217 This would thus be one of uncounted examples showing that there is no objective criterion to distinguish between homologies (descent-related similarity) and convergences (independently originated traits). The discovery of Haplocheirus, classified as a basal alvarezsaurid (Choiniere et al. 2010), provided strong support for this classification.
Two new Lower Cretaceous alvarezsaurid finds, Xiyunykus and Bannykus, are intermediate in the construction of their forelimbs, but also stratigraphically, between the Upper Jurassic genus Haplocheirus and the Upper Cretaceous alvarezsaurids with their highly reduced forelimbs. Describers Xu et al. (2018) see these finds as transitional stages in the evolution of alvarezsaurids. However, Bannykus shows only a slight proportional reduction of forelimbs compared to Haplocheirus, while the same is true for Xiyunykus. However, typical features of parvicursorines (Upper Cretaceous subgroup of alvarezsaurids) and special features218 are also developed on the forelimbs (Xu et al. 2018, 5). If the two new genera are interpreted as transitional forms, they support the interpretation that the avian features of the alvarezsaurids are convergences.
All interpretations for evolutionary classification are therefore quite problematic and this results from the conceptual default of evolution. Without this presupposition Mononykus can be interpreted as a special mosaic form without ancestral connection. The evolutionary theoretical problem is underlined by the fact that such a remodeling of the anterior extremity is problematic in all discussed directions. Novas and Pol (2002, 121) discuss the critical objection that the forelimbs of Mononykus cannot be wing precursors because the “morphological difference” is too great, and suggest that this argument also speaks against a relationship of Mononykus to ornithomimids, maniraptorans, coelurosaurs, theropods, or even dinosaurs. The cladistic analysis is considered decisive, while functional considerations are secondary (Novas and Pol 2002, 122). Such reasoning is highly questionable, however, because cladistic analyses do not clarify evolutionary mechanisms, as a suitable cladogram may very well be opposed by a functional impossibility. The omission of functional aspects is decidedly unbiological and therefore to be rejected.
The example of Mononykus shows that the existence of avian characteristics does not necessarily mean that the animal in question actually has a closer phylogenetic relationship to birds.
Avimimus
The Upper Cretaceous oviraptorosaurid genus Avimimus (fig. 60), an approximately 1.5-meter-long, bipedal walking theropod with a relatively small skull and largely toothless beak, is described as “enigmatic” by Vickers-Rich, Chiappe and Kurzanov (2002). Avimimus combines some unusual bird-like features with those of more primitive coelurosaurs and some peculiar specializations (Clark, Norell, and Makovicky 2002, 38; see also Molnar 1985, 213f.; Zhou 1995, 962). It is one of the most avian-like oviraptorosaurids, and it is characterized by many unusually avian-like features, including a toothless jaw (although some small teeth are preserved in some individuals), a protruding antitrochanter of the hip joint, and fusion of various skeletal elements, including the cranial roof, carpometacarpus, synsacrum, tibiotarsus, and tarsometatarsus. The shape of the pelvis indicates that the tail was long. With the extremely long and slender legs, Avimimus was probably a highly specialized runner. The discoverer S. M. Kurzanov reconstructed Avimimus with well-developed feathers, for which he cited elevations on the ulna as indirect evidence (these could be attachment points for arm wings), but this interpretation is controversial (Funston et al. 2016, 1).
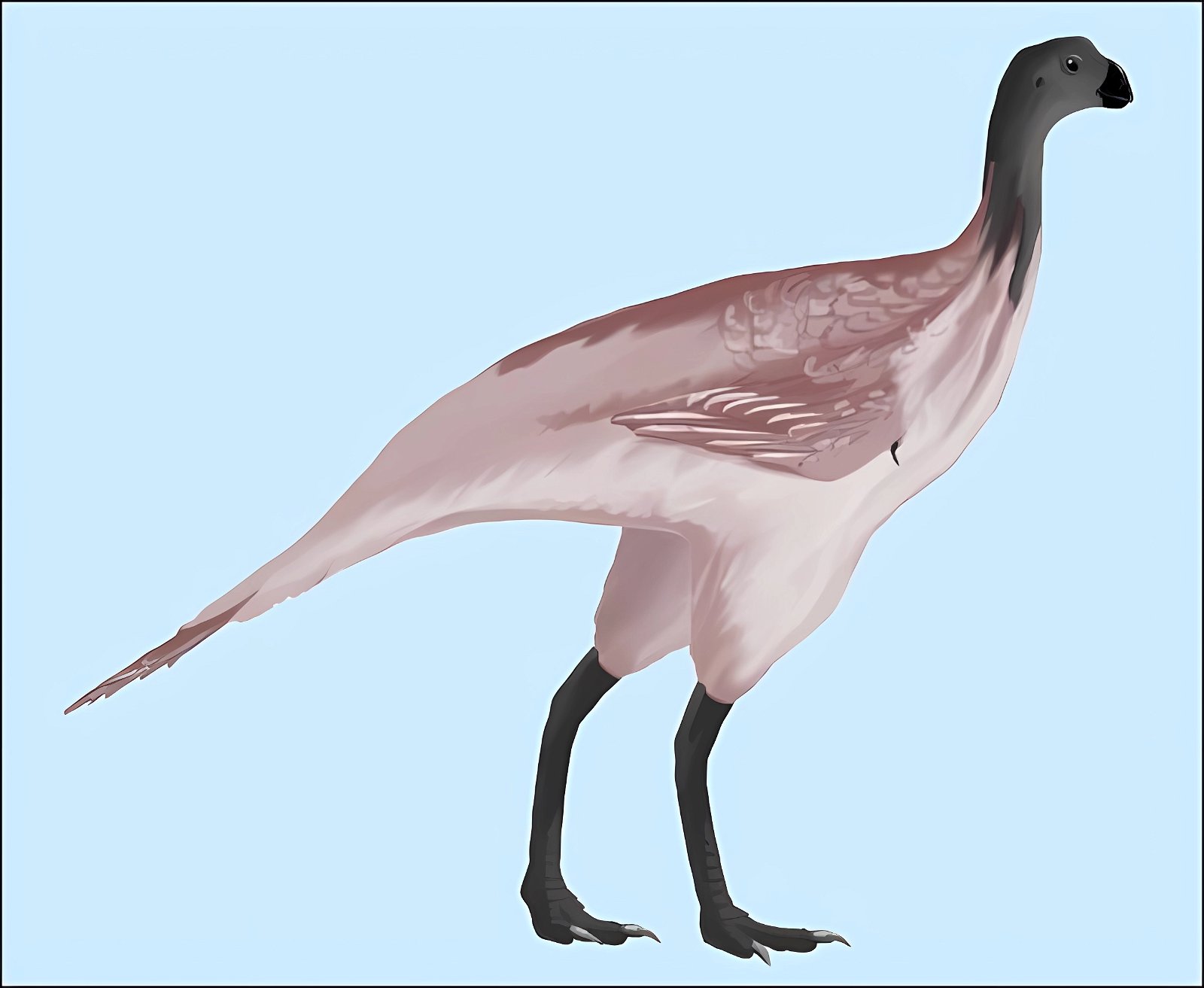
Fig. 60. Reconstruction of Avimimus portentosus. Matt Martyniuk, CC BY-SA 3.0.
For Vickers-Rich, Chiappe, and Kurzanov (2002, 65), the phylogenetic position of Avimimus is a “puzzle that remains to be solved.” This genus has been considered a theropod dinosaur close to the origin of birds (with some convergently evolved avian features) or a secondarily flightless basal bird (Vickers-Rich, Chiappe, and Kurzanov 2002, 65). The stratigraphic position in the Upper Cretaceous does not fit either alternative. Norman (1990) concluded that Avimimus was an unusual theropod dinosaur with a highly idiosyncratic combination of features, some of which are found in other groups of theropod dinosaurs or in birds.219 There were features pointing to theropod dinosaurs, others to sauropods, ornithopods, tetanurans, ornithomimids, or birds. Holtz (1994) placed Avimimus in a taxon he established, Arctometatarsalia, which is not closely related to the origin of birds. According to Holtz, Arctometatarsalia includes all Coelurosauria except those more closely related to birds. This taxon is considered obsolete today and is barely used.
Tsuihiji et al. (2017) used additional finds with previously unknown details to show that the head morphology of Avimimus is not as avian as previously thought, but intermediate between Early Cretaceous oviraptorosaurs and the diverse oviraptorosaurian subgroup Caenagnathoidea.
Incisivosaurus
Oviraptorosaurs are an unusual group of theropod dinosaurs with highly specialized skulls. As discussed above, numerous features are developed in these forms that associate them more with birds rather than with theropod dinosaurs. The Oviraptorosauria genus Incisivosaurus (fig. 61) is interesting in that it is closer in some features to other theropod groups than more derived forms. Thus, it narrows the morphological gap to other groups.220 The skull is low and the jaws are dentate. Since Incisivosaurus is at the same time one of the geologically oldest genera of this group (Lower Cretaceous, Barremian), the phylogenetically reconstructed position also fits stratigraphically (Xu et al. 2002a).
Fig. 61. Reconstruction of Incisivosaurus. Tomopteryx, “Incisivosaurus reconstruction by Tom Parker (Tomopteryx/Tomozaurus),” https://en.wikipedia.org/wiki/Incisivosaurus#/media/File:Incisivosaurus_(pencil_2013).png. CC BY-SA 3.0.
The teeth of Incisivosaurus, however, are not intermediate in formation, but are markedly specialized. One pair of premaxillary teeth is reminiscent of rodent teeth known in mammals, while the molars were lanceolate with large wear surfaces, features otherwise unknown in Theropoda and indicative of an herbivorous diet (Xu et al. 2002a). The pencil-shaped premaxillary teeth are comparable to the dentition of some herbivorous sauropod dinosaurs, the lanceolate molars resemble those of therizinosauroids (Xu et al. 2002a, 293), and connections to other groups appear reticulate in dentition features.
The differentiation of the dentition is much more pronounced in Incisivosaurus than in other theropod dinosaurs.
From an evolutionary theoretical perspective, this genus supports the interpretation that oviraptorosaurs were not derived from birds but acquired their numerous avian traits convergently (Xu et al. 2002a, 292; see Balanoff et al. 2018, 126221). The overall view that follows is: On the one hand, in Oviraptorosauria and Alvarezsauridae, the derived and stratigraphically younger members are more bird-like than the basal taxa in a number of characters,222 while on the other hand, in other lineages such as Dromaeosauridae and Troodontidae, the derived members are less bird-like, which is interpreted by reversion to more primitive characters. “This leads to conflicting results in reconstructing the phylogeny of maniraptorans and highlights the importance of including basal members of each group when attempting to reconstruct the phylogeny” (Xu et al. 2002a, 292f.).223
Unenlagia
The dromaeosaurid Unenlagia (fig. 62) was about 2 m long, only fragmentarily preserved (the skull is missing completely) and can be placed in the morphological gap between Archaeopteryx and dromaeosaurids in some features (Norell and Makoviocky 1999; Novas and Puerta 1997). Several features are more bird-like than in any other theropod dinosaur known to 1997. Unenlagia resembles Archaeopteryx in morphology of the scapula, pelvis, and legs, while several features of the pubis, ischium, and proportions of the hind leg bones were primitive and therefore suggest that Unenlagia may be the sister taxon of Avialae. Remarkably, the construction of the forelimb suggests that folding was possible in a bird-like fashion and that the ability to flap the forelimb upward, necessary for active flight, was already present in bipedal, non-flying theropod dinosaurs (Novas and Puerta 1997, 390).
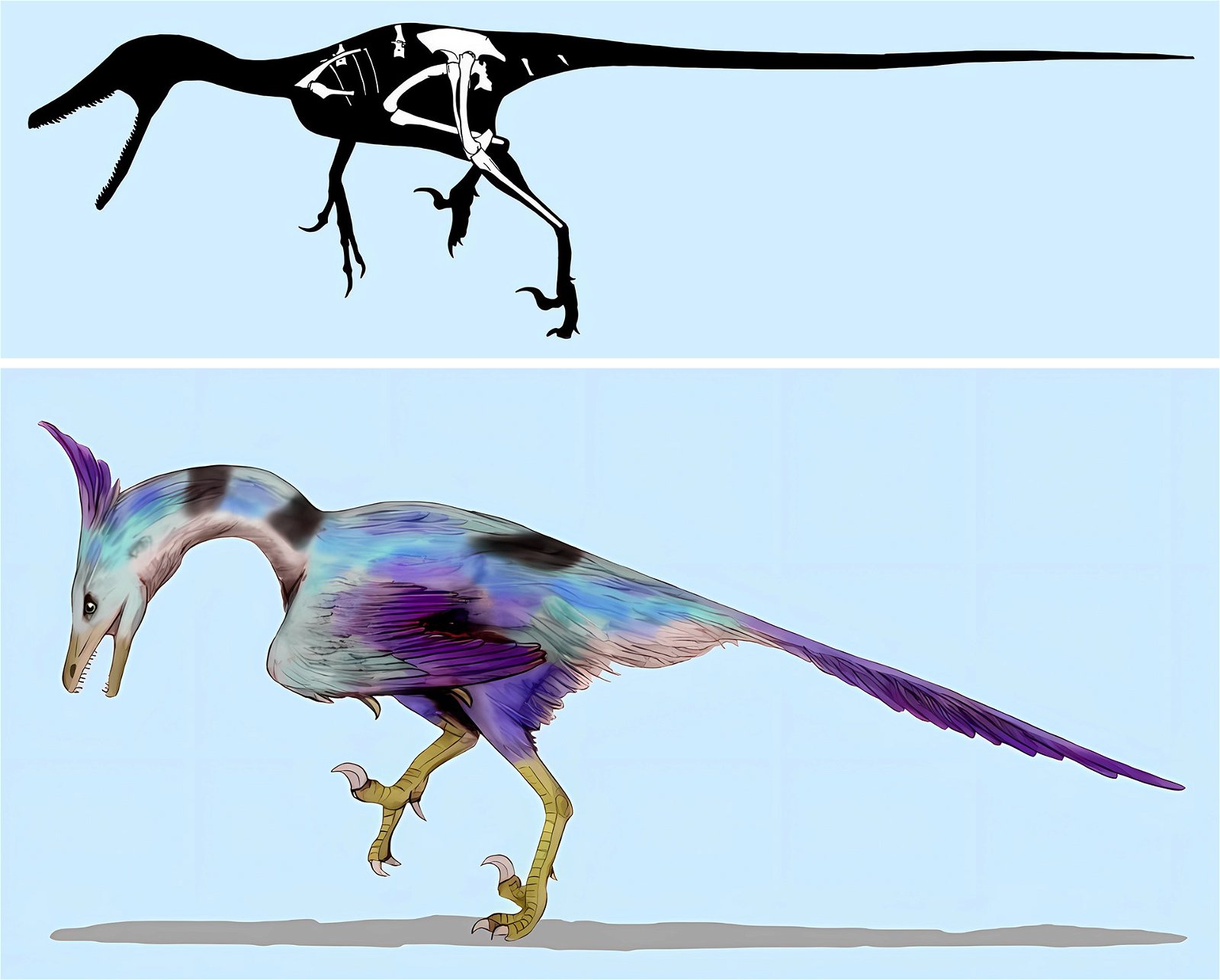
Fig. 62. Reconstructions of Unenlagia. (Top: Jaime A. Headden, “Skeletal reconstruction of Unenlagia comahuensis,” https://en.m.wikipedia.org/wiki/File:Unenlagia.jpg. CC BY 3.0. Bottom: Nobu Tamura, “ Unenlagia comahuensis, a dromaeosaur from the Late Cretaceous of Argentina, pencil drawing, digital coloring,” https://en.wikipedia.org/wiki/File:Unenlagia_BW.jpg, CC BY 3.0.
Novas and Puerta (1997, 391) consider that the size of Unenlagia combined with short forelimbs argues for flightlessness, but that the phylogenetic position argues against Unenlagia being descended from flightless forms. However, Unenlagia is not fossilized until the Upper Cretaceous (Turonian-Coniacian, ~90 Ma). Thus, there is a clear discrepancy between morphology and stratigraphy.
Eoalulavis
The enantiornithine genus Eoalulavis (fig. 63) is notable for documenting a surprisingly early formation of the bastard wing (alula) (Sanz et al. 1996; Sanz et al. 2002). It “illustrates the surprisingly early diversity and complexity of flight adaptations” (Shipman 1997, 31). The basic structure of the modern flying machine had been formed, making it possible to fly at low speeds and with high maneuverability (Sanz et al. 1996, 442).224

Fig. 63. Reconstruction of Eolalulavis. © Eloy Manzanero, dinodata; http://dinodata.de/animals/birds/pages_e/eoalulavis.php, CC BY 4.0.
In addition, Eoalulavis had a particular mosaic of features—the sternum is unusually narrow, spear-shaped, and possesses a weakly developed keel, the furcula is robust, the shoulder girdle is more “modern” than in the related species Iberomesornis, but the fingers possessed primitive claws of unknown function (Sanz et al. 1996; Shipman 1997, 31). The morphology of the foreleg in Eoalulavis is remarkably primitive compared to other enantiornithine genera (O’Connor, Chiappe, and Bell 2011, 82ff.). Although many inferred characters readily permit assignment to Enantiornithes, the precise relationships are unclear (Sanz et al. 1996, 443).225
Ambiortus
The approximately crow-sized Ambiortus (fig. 64) is one of the geologically oldest ornithuromorphs. This genus exhibits a “mosaic of archaic and specialized features” (Kurochkin 1985, 271). Some features relate it to “more highly evolved” birds from the palaeognath group (Kurochkin 1985) and the Ichthyornithes (Kurochkin 1999). Thus, Ambiortus possessed a keeled sternum and a bird-like shoulder girdle (Kurochkin 1985, 272).226 This author distinguishes three types of features in Ambiortus—generalized features typical of true birds, features it shares with certain groups of fossil and some modern birds, and unique features that require a new taxon of high rank.227

Fig. 64. Reconstruction Ambiortus dementjevi. Scott Reid, https://a-dinosaur-a-day.com/post/167928034680/ambiortus-dementjevi.
Most analyses identify this genus as belonging to the ornithurans, but without being able to position it more precisely in this group, or as a primitive genus of the ornithuromorphs. O’Connor and Zelenkov (2013, 1270) state, “In general, the mosaic pattern of character distribution among early ornithuromorph taxa does not reveal obvious relationships between taxa.”
Protopteryx
The approximately starling-sized enantiornithine Protopteryx (fig. 65) is one of the geologically oldest opposite birds and is also classified as primitive (for example, the fibula is not greatly reduced, there is no fused tibiotarsus, the hand bones are relatively long, the thumb bone is long, and the external carpal bones and metacarpals are not completely fused into the carpometacarpus). The genus has many skeletal transitions between Archaeopteryx and Confuciusornis and more advanced birds according to Zhang and Zhou (2000, 1955). At the same time, Protopteryx also had special and more derived features, most notably two long scale-like tail feathers that were unbranched in the region near the body, an otherwise unrecognized feather type (Wang et al. 2014; as discussed above). The number of teeth appears to be greatly reduced. With modern birds, Protopteryx shares a process on the procoracoid process and a lateral process of the coracoid. In addition, a bastard wing (alula) is developed as a “modern” feature (although Zheng et al. [2017, 448] suggest this feature is a common inheritance of Ornithothoraces).
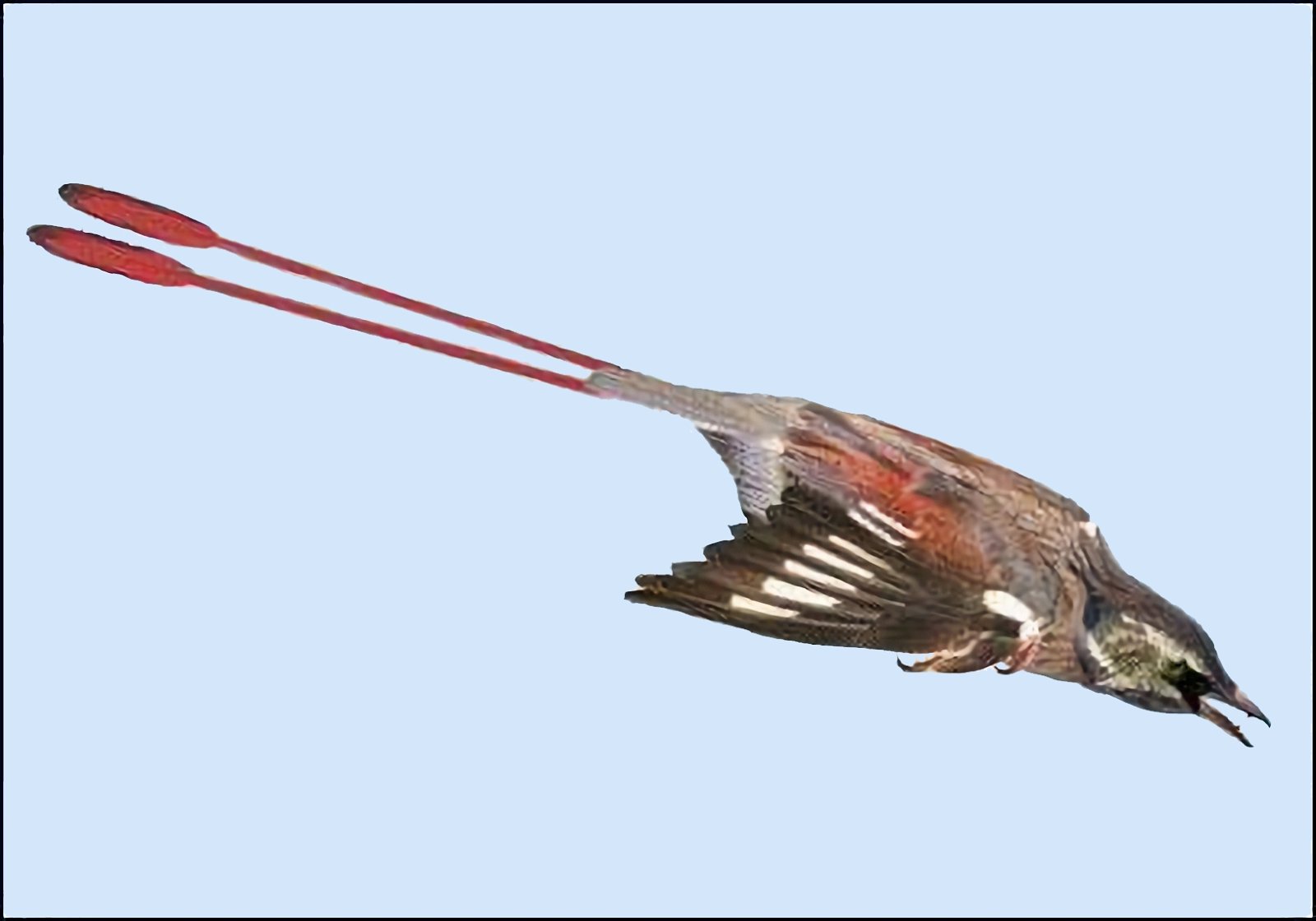
Fig. 65. Reconstruction of Protopteryx. es.dino.wikia.com, CC-BY-SA.
Geologically even slightly older is Eopengornis, which along with the other pengornithids is placed on a much more derived position within Enantiornithes (Hu, O’Connor, and Zhou 2015).
Archaeorhynchus
Another remarkable discovery was made in the ornithuromorph Archaeorhynchus spathula (fig. 66). The ornithuromorphs (“bird tails”) are the group of birds from which today’s birds are thought to have evolved, and they include them. Their fossils occur in considerable diversity since the Lower Cretaceous. Archaeorhynchus spathula from the Lower Cretaceous of China (Jehol Formation, dated to 120 million radiometric years) was about the size of a starling and is classified as “basal” based on skeletal features, although this species also has a number of “modern” avian features (a toothless beak, sternal quill, furcula shape, and asymmetrical feathers). Of note is the preservation of soft tissue, which Wang et al. (2018a) interpret as fossilized lungs, which they consider a likely interpretation. Wang et al. (2018a) conclude from the fossil remains that the lungs were “very similar to the lungs of present-day birds.” The specializations of the lungs, such as the highly subdivided parenchyma, were “modern.” The researchers found structures they thought resembled parabronchi (which are very fine capillaries in the lungs of today’s birds). These features indicated that the amount of oxygen required for powered flight could be absorbed in this species. The fossil evidence further showed that the lungs were fixed directly to the wall of the cavities, as in modern birds, making the lungs virtually immobile, as is the case in modern birds. This fixation makes it possible for the parenchyma to be divided into extremely fine capillaries without collapsing the tissue, allowing for a large airway area and the development of an extremely thin blood-gas barrier (Wang et al. 2018, 11559).

Fig. 66. Artistic representation of Archaeorhynchus spathula, which was slightly larger than a dove. Brian Choo, “An artist’s interpretation of the dinosaur-era bird Archaeorhynchus spathula, which was a bit larger than a modern pigeon,” https://carnivora.net/archaeorhynchus-spathula-t3783.html.
The authors see this finding in a set of features on soft tissue systems (for example, digestion and respiratory system) that are typical of birds living today and were established early, before changes in the skeleton occurred.228 In contrast, the skeletal features associated with respiration were primitive.
Features of the tail are also unusual. In addition to the ordinary tail feathers, two particularly long, narrow tail feathers protrude above the rest of the feathers (called a “pintail”). This type of feather was previously unknown in Cretaceous birds, but occurs in present-day birds as in some species of hummingbirds.
Evaluation
All in all, Archaeorhynchus shows a distinct mosaic of features, which hardly fits into an evolutionary transitional position. And once more it shows that requirements for flying, here bird-typical efficient lungs, were developed from the beginning.
Orienantius
Another species to be presented here is placed in the genus Orienantius from the opposite birds (Enantiornithes).229 It belongs to the geologically oldest opposite birds and was discovered in the Huajiying Formation of China, which is dated to 131 million radiometric years. Soft tissues are also fossilized and preserved in this find, namely parts of the flight skins (patagia). Liu et al. (2018, 16) write, “The morphology of the preserved soft tissues shows that the main patagia (propatagium, postpatagium, and metapatagium characteristic of the wings of modern birds were already present in the earliest known enantiornithines.”230 (fig. 67) Their expression suggests that these birds could maneuver very well and alternately flap and glide, comparable to present-day small and medium-sized birds (Liu et al. 2018, 17).
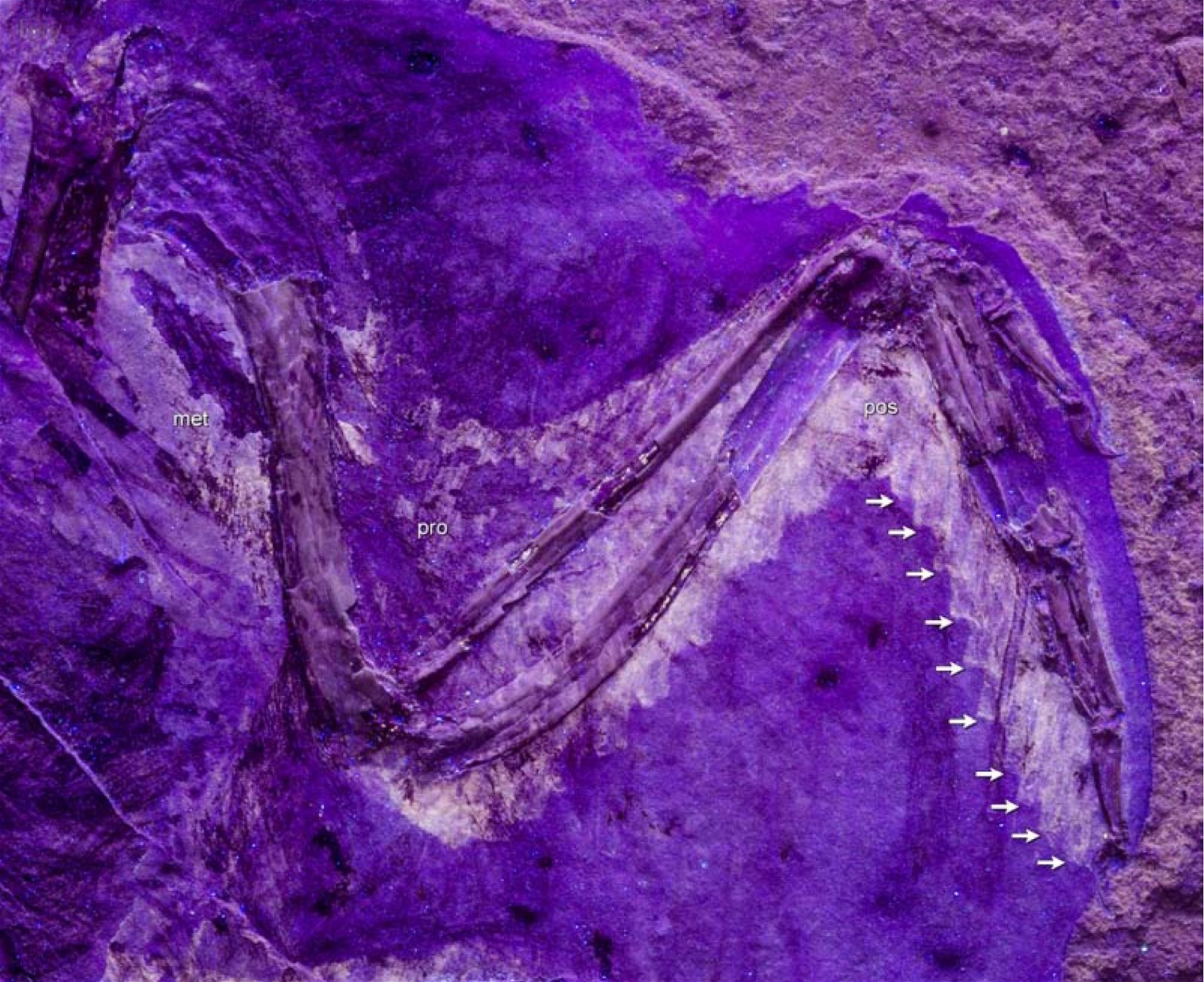
Fig. 67. Detail of the anterior extremity of Orienantius ritteri under UV light. White arrows point to the base of the calami of the ten primary hand wings in the postpatagium. Abbreviations: met metapatagium, pos postpatagium, pro propatagium (from Liu et al. 2018, with kind permission).
Several years ago, Navalón et al. (2015) had already detected flight skins on an unnamed opposite bird, which they interpreted as evidence of good flight skill (summarized by Junker [2015]).
The researchers further demonstrated that the preserved contour of the musculature of the leg is identical to that of modern birds. The soft tissues around the foot bones indicate that the feet of Orienantius ritteri had no major muscles, mirroring the situation in birds living today (Liu et al. 2018, 16).
Evaluation
In the case of Orienantius, it is also evident that good flight ability, as well as other avian-typical traits, are expressed within the opposite birds from the beginning, in the geologically oldest forms. Liu et al. (2018, 18) conclude that this new taxon exhibits numerous features that are typical of later taxa, and that the opposite birds already exhibit a significant degree of taxonomic differentiation in their earliest appearance in the fossil record.
Jinguofortis
Another example of “chaotic” evolution (Min Wang231) is the approximately crow-sized fossil species Jinguofortis perplexus, also described in 2018 (fig. 68). Its systematic position is not clearly determined. It is placed in the newly erected family Jinguofortisidae along with the similarly enigmatic genus Chongmingia (Wang et al. 2016; Wang, Stridham, and Zhou 2018). Because it possessed a pygostyle (fused caudal vertebrae) like modern birds, it is included in the pygostylians, which include all birds except the famous Archaeopteryx and Jeholornis (these two genera possessed a long caudal spine and, correspondingly, a pinnate tail that was constructed differently from the fan-shaped tail usually formed in birds). Another distinctly “modern” feature is the greatly reduced fingers.

Fig. 68. Reconstruction of Jinguofortis perplexus. © Chung-Tat Cheung.
However, in addition to bird-typical features, features otherwise known in theropod dinosaurs anduntypical of birds also occur. These include claws on the fingers of the wings, a boomerang-shaped, presumably rigid furcula (clavicle),232 a jaw with tiny teeth instead of a beak, and a fused shoulder girdle (scapula and coracoid). Since the latter feature appears unfavorable for flying because it limits flexibility for flapping flight, the species received its species name “perplexus.” Nevertheless, the broad, short wings of Jinguofortis were typical of birds that could maneuver well among trees. Perhaps a previously unknown mode of flight was realized. In modern birds, a comparable fusion of the two shoulder joint bones is known only in flightless forms. However, that Jinguofortis was also secondarily flightless seems rather unlikely, especially since the wing feathers were distinctly asymmetrical (Wang, Stridham, and Zhou 2018, 10710).
The fused shoulder girdle (scapula and coracoid) is similar to the situation in some theropod dinosaurs, but nevertheless this feature is not suitable as evidence for a phylogenetic link between dinosaurs and birds, because in Archaeopteryx these two bones were not fused. Therefore, an independent (convergent) origin is assumed, and moreover, also convergent in Confuciusornis (Wang et al. 2016, 2018b). Wang et al. (2018b) suggest that accelerated bone formation is causative.
The possession of teeth is common in Mesozoic birds, and also with respect to this feature the expression in Jinguofortis does not fit well from an evolutionary theoretical point of view. Because in Jinguofortis premaxilla and maxilla were toothed, while in Archaeopteryx and the Ornithothoraces (Enantiornithes [opposite birds] and Ornithurae [“bird-tails”]) the tip of the premaxilla and in Jeholornis the whole premaxilla is toothless. Therefore, with respect to this feature, evolutionary theory would have to assume a regression or, as with the shoulder girdle, a kind of evolutionary zigzag course, which is generally considered implausible.
Evaluation
Wang et al. (2018b, 10708) note that Jinguofortis increases the known diversity of early pygostylians and suggest that developmental plasticity played an important role and that putative evolution is mosaic.233 (Plasticity is the ability to express traits differently depending on environmental influences and has nothing to do with evolution per se.) The Jinguofortisidae contribute to the widespread occurrence of mosaic evolution (Wang, Stridham, and Zhou 2018, 10710). Brusatte comments in National Geographic on this finding that there has been much “experimentation”.234 However, both mosaic evolution and experimentation are foreign bodies in an evolutionary scenario. As experimentation is a purposeful process, the term obscures a finding that is unexpected in evolutionary theory. Trait distributions do not fit into a hierarchical nested system. Therefore, convergences must be assumed to a large extent, which is called “mosaic evolution.” But why and by what means does a natural, future-blind evolutionary process arrive at similar constructs many times independently? Moreover, the mosaic of features is such that it does not fit altogether as an evolutionary transitional form, but must be assumed to be an evolutionary lineage of its own.
Conclusion
Further genera could be cited here. However, the discussed examples already give a good impression of the frequently occurring feature contradictions, which require the assumption of numerous convergences in an evolutionary-theoretical interpretation, that is, in a tree representation of the relationships.
Summary and Evidence for Creation
- The findings compiled here in this paper show that the similarity relationships of the diverse forms of Jurassic and Cretaceous theropod dinosaurs and avian genera are more reticulate than tree-like. One author even uses the term “chaotic” (Brusatte 2017a, 792). This can be seen in the following points:
- It is not clear which group is at the base of the birds (Brusatte et al. 2014, 2386; Currie 1997, 230; Xu et al. 2014, 1).235
- As a rule, a multiple independent (convergent) origin of bird-typical traits must be assumed and, depending on the underlying trait, partly different relationships are suggested (see fig. 1). For example, Kurochkin (2006, 45) notes that bird-like traits occur in various combinations in dromaeosaurs, troodontids, oviraptorids, therizinosaurs, and tyrannosaurs, but that no group of coelurosaurs possesses the complete set of bird-like traits. This suggests that theropods and birds acquired them in parallel.236 The large extent of homoplasy means that cladograms are not stable and new finds can lead to major changes (Leigh 2014, 2; see also Feduccia 2012, 188).237
- There is controversy in some genera or entire groups as to whether they are avian precursors or secondarily flightless birds.
- The oldest bird groups are already strongly differentiated at the base and there are partly also “modern” characteristics in the oldest representatives of a group.
- Especially in plumage characteristics, the greatest degree of diversity is seen near the base of the bird groups.
- Most theropod dinosaur genera possessing bird-like features are geologically younger than the geologically oldest birds. The genera with avian features are in many cases fossilized only from the Upper Cretaceous, when a great diversity of avian forms had long been established (Kurochkin 2006, 46238; see also Dodson 2000, 504f.239; Currie 1997, 230; see figs. 4, 5).
In a review paper, Agnolin et al. (2019, 22) conclude that a consensus on the phylogenetic relationships of Paraves is far from being reached. Therefore, they argue, knowledge about the steps involved in the acquisition of anatomical and behavioral traits that contribute to the origin of avian flight is still uncertain and in flux. The authors further state that they could not find a clear sequence of evolutionary innovations. Also, they say, the fossil record is sparse in the area of the transition from dinosaurs to birds, and new fossils are needed to fill in the gaps between the various clades of Paraves.240 The nature of the early radiation of Paraves and birds is “completely uncertain,” as are their center of origin and dispersal routes, they said.241 If evolution had really occurred, one would not expect such a result (“totally uncertain”).
“Experiments”
This situation regarding early trait diversity and presumed blind evolutionary ends prompts numerous authors to speak of evolutionary “experimentation.” For example, Sullivan, Xu, and O’Connor (2017, 1, 3) speak of “rapid diversification of aerodynamic structures” in Paraves, showing a “surprising amount of homoplasy and evolutionary experimentation.” The scansoriopterygids have been called an “evolutionary experiment near the base of the avialan tree” (Sullivan et al. 2014, 269).242 Also for O’Connor, Chiappe, and Bell (2011, 49), some Cretaceous birds show an “unforeseen quantity of evolutionary experimentation and homoplasy,” on the one hand with typical features of maniraptorans that are absent in Archaeopteryx, and on the other hand also features that were more primitive than in Archaeopteryx, although phylogenetically closer to modern birds. The origin of all major groups was accompanied by a “wide range of evolutionary experimentation,” as Chiappe (2009, 248) also suggests. Closely related lineages, whether coexisting or not, would thereby converge to a greater or lesser extent on the characteristic features of the new group. Glaubrecht (1998, 36) makes a similar point: “Some primordial birds are but witnesses to abandoned experiments in evolution.” The evolution of flight was chaotic, Brusatte (2017a, 792) was quoted at the outset, with different dinosaurs supposedly experimenting with different flight behaviors and with different wing and feather arrangements.243 Many lineages of the Paraves supposedly experimented with different modes of flight during the Upper Jurassic and Lower Cretaceous, according to Puttick, Thomas, and Benton (2014, 1497).244
It has been claimed for a long time that much apparent experimentation takes place and many convergences occur when a new “adaptive zone” is reached, Agnolin et al. (2019, 22) also state. Therefore, they argue, the adaptive breakthrough of the evolution from dinosaurs to birds is accompanied by numerous convergences in closely related lineages in bird-like traits. The degree of evolutionary experimentation and convergences in “avianness” highlighted by recent discoveries, particularly from China, meant that consensus on phylogenetic relationships remained elusive.245 Further statements of this sort could be cited.246
However, this situation is problematic in evolutionary theory for the following reasons:
- Classically, evolutionary theory predicts that the diversity of forms can be represented in a reasonably consistent tree-like fashion. The tree-shaped representation has often been inversely interpreted as evidence for evolution. Strongly reticulate or even chaotic trait relationships go in the direction of the opposite of what is predicted by evolutionary theory. Further findings cannot unravel this network either, but they could strengthen it.247
- Strictly speaking, one can only speak of “experiments” if there is also an experimenter. Since this does not exist in evolution, this frequently used term is misleading. It suggests a steering, because an experimenter, who tries out different things, pursues a goal. But evolutionary processes as pure natural processes cannot do that. Ultimately, the “experiment” metaphor conceals a problem in evolutionary theory. For even if this term is of course meant metaphorically, it should represent a reality. But this is just not the case. If then also failed experiments are mentioned (for example, in the case of the four-winged forms), a goal orientation is also implied, because failure can only be spoken of in relation to a goal.
- Both Enantiornithes and Ornithurae appear relatively abruptly in the fossil sequence in great diversity, temporally in common with forms such as Confuciusornis, Jeholornis, and Sapeornis, which are classified as more primitive. Although there are partial trends within individual groups, this sudden appearance is striking.
- Mismatched mosaic forms lead to dilemmas or trilemmas (Alvarezsauridae) when evolution is assumed.
Ghost Lineages
The cladogram published by Foth and Rauhut (2017) shows that for a large part of the coelurosaurs long ghost lineages have to be assumed (fig. 69). That is, it must be assumed under evolutionary theoretical conditions that many lineages left no fossils during 20–30 million years of their assumed existence and in some cases even more, while fossil remains of other lineages in comparable geological strata have survived. Such a situation is problematic in evolutionary theory and in a long-term framework.
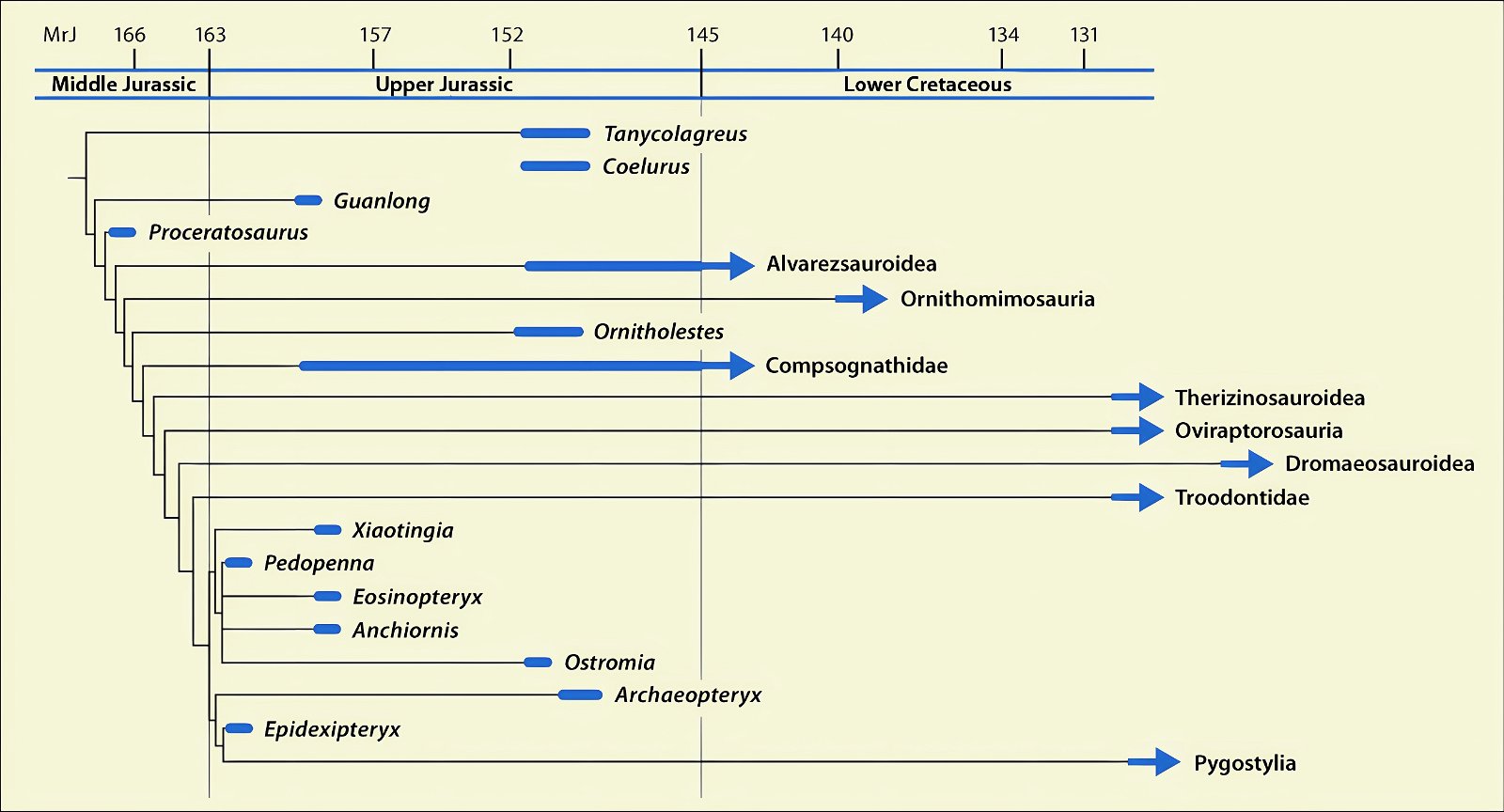
Fig. 69. Simplified phylogeny of maniraptorans. According to Foth and Rauhut (2017), the stratigraphic and geographic distribution indicates rapid radiation in the late Middle to earliest Late Jurassic in East Asia (according to Foth and Rauhut 2017; simplified).
Anomalies for Evolution and Evidence for Creation
In the questions on origins in biology usually only explanations are discussed which are committed to the naturalistic paradigm. That means only (purely) natural, blind, non-spiritual processes are allowed, which can be described lawfully (if necessary by statistical regularities), as well as random boundary conditions are admitted. This determination leads logically to an evolutionary approach. Accordingly, an attempt is made to represent the diversity of life-forms in a tree-like manner and to prove an essentially gradual variation. For the basic mechanisms of evolution are descent and speciation with usually dichotomous branching of parent species into two daughter species. Horizontal inheritance (from one species into another), which does not proceed via sexual reproduction, is almost unknown in multicellular organisms and therefore cannot be taken into account.248
Since, according to all that is known about evolutionary mechanisms, the evolutionary process is not goal-directed249 (which also corresponds to the naturalistic paradigm), according to evolutionary theory it was expected that convergences would be rare and all the more unlikely the more complex the structures in question are. Accordingly, it was expected that the diversity of forms could be easily represented in tree diagrams. Cladism is based on these expectations. Indeed, if convergences were expected to occur easily and therefore to occur frequently, cladism would be methodologically at an end. However, the overwhelming majority of biologists do not see it that way.
In addition, it is clearly to be expected from evolutionary theory that forms, which are classified as “primitive” (see table 1) appear in the fossil sequence stratigraphically (in evolutionary theory interpretation also temporally) before the more derived forms, at least as a tendency, as soon as the “sample” is sufficiently large (that is, a larger variety of forms is fossilized). Furthermore, it follows from the effect of the evolutionary mechanisms that a sequence of increasing diversity can be found in the sedimentary sequences.
The comments made here have shown that all these expectations have been met only to a limited extent or almost not at all (see table 4):
- Convergences are extremely frequent. Accordingly, the cladograms usually have low consistency indices.
- A representation by tree diagrams is always possible, but the concrete form is sometimes controversially discussed and often had to be changed by new fossil findings.
- Accordingly, the groups of shapes could be arranged in the form of networks on the basis of the feature distributions, but this is not practiced because it does not correspond to the underlying approach.
- The diversity of forms of Mesozoic birds occurs explosively in the Lower Cretaceous in considerable diversity.250 The Jurassic birds or genera that are morphologically close to the birds are hardly suitable as possible precursors or only with reservations.
- The majority of dinosaurian genera with apparent avian characteristics are geologically dated younger than a large number of opposite birds and ornithuran birds and the basal avian genera Archaeopteryx, Confuciusornis, Jeholornis, and Sapeornis.
- Some genera classified as relatively derived are among the oldest of their respective groups (this is the case with both Enantiornithes and Ornithurae).
- A number of bird-like features appear abruptly. Although many of them seem to be found in some genera of theropod dinosaurs, in these they are partly distributed unsystematically (different groups show different bird characteristics) and convergent evolution must be assumed, which occurred only after the establishment of the birds (with which in these cases the features in question cannot be precursor features).
- The known evolutionary mechanisms seem to be clearly overwhelmed by the need to produce a large variety of forms relatively abruptly in geologically short periods of time.251
Table 4. Evolutionary theoretical expectations and observations on the putative evolution of birds from dinosaurs.
| Evolutionary expectations | Observations |
|---|---|
| Convergences are rare and less likely with increasing complexity. | Convergences are extremely frequent. |
| Shape diversity is easily represented in tree diagrams (cladism). | Cladograms are unstable, often changed, controversial, reticular relationships. |
| “Primitive” forms are (tend to be) recorded fossil before derived forms. | Relatively derived forms at base, presumed ancestors partly “younger” than descendants. |
| Fossil sequence shows increasing diversity in time. | Fossil sequence shows explosive occurrence. |
| Known mechanisms explain the way of occurrence of the diversity of forms. | Known mechanisms do not explain sudden occurrence. |
Alternatively, if we assume a creation of basic types (Scherer 1993), that is, created kinds, the above findings can be explained as follows:
- The explosive occurrence of the variety of forms reflects the existence of basic types, which were created in finished distinct and diverse form.
- The numerous, most different mosaic forms are expression of manifold combinations of characteristics, whose expression is explained by the respective way of life and not by a preceding evolution. A creator is free in his actions, while by evolution can be rebuilt only gradually (from which the expectations mentioned above follow, but did not come true).
- The difficulties (if not aporias) that arise for evolutionary theoretical modeling of how the various mosaic forms arose are unnecessary if the traits can be freely combined according to the requirements for particular lifestyles and habitats.
- The discussed problem of an “experimentation” is omitted. There is no “experimentation,” but an initial variety of forms, which was originally in some respects the greatest (especially for feather types and flight forms).
- The question of evolutionary mechanisms for rapid and diverse occurrence of a wide variety of forms is not applicable.
However, some findings appear to be interpreted well from an evolutionary theoretical point of view. Some mosaic forms could appear to be close to evolutionary transitional forms. However, most mosaic forms are not suitable for this, because their feature combinations do not fit into basal positions of groups. In some groups, trends can be traced throughout the Cretaceous (for example, different expressions of beaks and different degrees of tooth reduction, as well as different pygostyle types).252 The fact that many apparent avian features occur in different theropod dinosaur groups could also be taken as a point for evolutionary interpretation, but with the limitations already discussed above.
Creation
Let us finish with some brief remarks about creation. By “creation” is generally meant a supreme spiritual causation. Spirit endowed beings (persons like us who can design and make machinery, or especially the Creator God of the Bible) have self-consciousness, value categories, thinking ability, set goals and pursue them deliberately, etc. They can visualize things, that is, imagine them mentally (intentionality), plan accordingly, select means to reach the goal, take obstacles and possibly detours into account, set intermediate steps and can in principle consider any number of requirements in advance and organize their actions accordingly. Intentionality also enables a subject to act creatively and technically. In doing so, it can anticipate technical means, which it has derived from the consideration and analysis of its objective as well as from its background knowledge, again as sub-objectives and systematically bring them to bear (Widenmeyer and Junker 2016).
Non-spiritual, purely natural processes cannot do all this. They are, so to speak, “blind” with respect to goals or the achievement of a goal by suitable means; thus, they have no abilities in goal setting, the analysis of goals with respect to the choice of means, and, accordingly, the systematic pursuit of goals. Whatever boundary conditions are given, things simply proceed according to the laws of nature. Explanations that are not based on intentionality, that is, purpose setting and deliberate choice of means, can only refer to three factors—laws of nature, (statistically qualified!) chance, and plausible boundary conditions.
Therefore, the characteristics of products of mental versus non-mental causation are usually very different and easy to distinguish (Romans 1:19–20).
Glossary
Derived: → Apomorphic.
Apomorphic: Designation for a trait or a trait state that is evaluated in evolutionary theory as being more highly developed or derived.
Carpale: One of the wrist bones.
Cladism: Method for the determination of relationships. Common → apomorphic features (→ synapomorphies) are taken as a basis, on the basis of which the relationships (ancestry relationships) of the taxa studied are brought into a tree diagram (→ cladogram).
Cladogram: → Cladism.
Convergence: Similar expression of structures of organisms unrelated in ancestry, which are interpreted in evolutionary theory as having arisen independently.
Enantiornithes: Opposite birds. Only in Cretaceous sediments is this fossil bird group found, whose special characteristic is the articulation between the scapula and the coracoid. On the shoulder blade there is a socket and on the coracoid bone a joint pin, the other way round to that in other bird groups. In Carpometacarpus, the third metacarpal bone extended outward beyond the length of the second metacarpal bone. Most species were dentate and were forest dwellers.
Exaptation: An existing feature is (additionally) used for a new, previously unnecessary purpose.
Furcula: wishbone; in birds and some dinosaurs, a forked bone element fused from the two clavicles.
Homoplasy: Collective term for → convergences, parallelisms, and reverse developments (reversions).
Ornithomimosauria: “Bird mimicking lizards;” dinosaurs from the theropod group that resemble ratites.
Ornithothoraces: Bird group that includes both the → Enantiornithes and the → Ornithurae.
Ornithurae: “Bird tails,” birds with a fan tail, to which today’s birds also belong, fossilized from the Lower Cretaceous upwards.
Opposite birds: → Enantiornithes.
Ornithuromorpha: Group consisting of the → Ornithurae and the two genera Patagopteryx and Vorona.
Oviraptorosauria: Group of dinosaurs within the → theropods. Avimimidae, Oviraptoridae and Caenagnathoidea with a very bird-like appearance are counted among them.
Paraves: Birds and their evolutionarily most closely related groups, usually the deinonychosaurs (Troodontidae and Dromaeosauridae) are included (see fig. 4).
Parsimony principle: Principle in the creation of → cladograms according to which as few → convergences as possible occur.
Patagium: Flying skin.
Phylogeny: Evolutionary reconstructed lineage based on cladistic analysis.
Plesiomorph: Designation for a trait or a trait state that is evaluated as primitive or original in evolutionary theory.
Pneumaticity: Presence of air spaces in bones.
Pygostyle: Bone formed by the fusion of several vertebrae at the end of the spine of birds.
Pygostylia: Birds that have a → pygostyle.
Synapomorphy: → Apomorphic feature common to two or more groups.
Stratigraphy: Stratigraphic description, sequence of geological sedimentary layers.
Taxon (pl. taxa): Generally, a unit of classification, whether species, genera, families, orders, or other groups of living things, usually regarded as units of descent.
Therizinosauria: Group of mostly large → theropod dinosaurs with very long claws on their forelimbs.
Theropods: Only fossil preserved bipedal mostly predatory dinosaurs; they are placed in the dinosaur subgroup Saurischia (see fig. 1). In evolutionary theory, they are considered almost unchallenged as the forerunners of birds, which is critically considered in this work. The theropods include from a cladistical perspective also the birds; however, this assignment is not followed in this paper, but rather “theropod dinosaurs” are spoken of in distinction to them. Which groups among the theropods are to be counted to birds is partly disputed (see the section “Avian Precursors or Secondarily Flightlessness?”).
Triosseal canal: Between the furcula, coracoid, and scapula in birds is a gap, the foramen triosseum (tri-bone canal, triosseal canal), through which runs a strong tendon connecting the small pectoral muscle (supracoracoideus muscle) to the humerus. This system is responsible for lifting the wing.
References
Agnolin, Federico L., Matias J. Motta, Federico Brissón Egli, Gastón Lo Coco, and Fernando E. Novas. 2019. “Paravian Phylogeny and the Dinosaur-Bird Transition: An Overview.” Frontiers in Earth Science 6, no. 252 (12 February). doi: 10.3389/feart.2018.00252.
Agnolín, Federico L., and Fernando E. Novas. 2013. Avian Ancestors: A Review of the Phylogenetic Relationships of the Theropods Unenlagiidae, Microraptoria, Anchiornis and Scansoriopterygidae. Berlin, Germany: Springer.
Agnolin, Federico L., Jaime E. Powell, Fernando E. Novas, and Martin Kundrát. 2012. “New Alvarezsaurid (Dinosauria, Theropoda) from Uppermost Cretaceous of North-Western Patagonia with Associated Eggs.” Cretaceous Research 35 (June): 33–56.
Altangerel, Perle, Mark A. Norell, Luis M. Chiappe, and James M. Clark. 1993. “Flightless Bird from the Cretaceous of Mongolia.” Nature 362, no. 6421 (15 April): 623–626.
Alonso, Patricio Domínguez , Angela C. Milner, Richard A. Ketcham, M. John Cookson, and Timothy B. Rowe. 2004. “The Avian Nature of the Brain and Inner Ear of Archaeopteryx.” Nature 430, no. 7000 (5 August): 666–669.
Balanoff, Amy M., Gabe S. Bever, Timothy B. Rowe, and Mark A. Norell. 2013. “Evolutionary Origins of the Avian Brain.” Nature 501, no. 7465 (5 September): 93–96.
Balanoff, Amy M., G. S. Bever, and Mark A. Norell. 2014. “Reconsidering the Avian Nature of the Oviraptorosaur Brain (Dinosauria: Theropoda).” PLoS ONE 9, no. 12 (December 10): e113559. doi:10.1371/journal.pone.0113559.
Balanoff, Amy M., Mark A. Norell, Aneila V. C. Hogan, and Gabriel S. Bever. 2018. “The Endocranial Cavity of Oviraptorosaur Dinosaurs and the Increasingly Complex, Deep History of the Avian Brain.” Brain Behavior and Evolution 91, no. 3 (August): 125–135.
Balter, Michael. 2015. “When Modern Birds Took Flight.” Science 348, no. 6235 (8 May): 617.
Barsbold, Rinchen, Philip J. Currie, Nathan Myhrvold, Halszka Osmólska, Khishigjav Tsogtbaatar, and Mahito Watabe. 2000a. “A Pygostyle from a Non-Avian Theropod.” Nature 403, no. 6766 (13 January): 155–156.
Barsbold, Rinchen, Halszka Osmólska, Mahito Watabe, Philip J. Currie, and Khishigjaw Tsogtbaatar. 2000b. “A New Oviraptorosaur (Dinosauria, Theropoda) from Mongolia: the First Dinosaur with a Pygostyle.” Acta Palaeontologica Polonica 45, no. 2: 97–106.
Benson, Roger B. J., Richard J. Butler, Matthew T. Carrano, and Patrick M. O’Connor. 2012. “Air-Filled Postcranial Bones in Theropod Dinosaurs: Physiological Implications and the ‘Reptile’-Bird Transition.” Biological Reviews Cambridge Philosophical Society 87, no. 1 (February): 168–193.
Benson, Roger B. J., and Jonah N. Choiniere. 2013. “Rates of Dinosaur Limb Evolution Provide Evidence for Exceptional Radiation in Mesozoic Birds.” Proceedings of the Royal Society B Biological Sciences 280, no. 1768 (7 October): 30131780.
Benton, Michael J. 2014. “How Birds Became Birds.” Science 345, no. 6196 (1 August): 508–509.
Bock, Walter J. 2013. “The Furcula and the Evolution of Avian Flight.” Paleontological Journal 47, no. 11 (11 December): 1236–1244.
Botelho, João Francisco, Luis Ossa-Fuentes, Sergio Soto-Acuña, Daniel Smith-Paredes, Daniel Nuñez-León, Miguel Salinas-Saavedra, Macarena Ruiz-Flores, and Alexander O. Vargas. 2014. “New Developmental Evidence Clarifies the Evolution of Wrist Bones in the Dinosaur-Bird Transition.” PLoS Biology 12, no. 9 (September): e1001957.
Botelho, João Francisco, Daniel Smith-Paredes, Sergio Soto-Acuña, Jingmai O’Connor, Verónica Palma, and Alexander O. Vargas. 2016. “Molecular Evolution of Fibular Reduction in Birds and Its Evolution from Dinosaurs.” Evolution 70. no. 3 (March): 543–554.
Britt, Brooks B. 1997. “Postcranial Pneumaticity.” In Encyclopedia of Dinosaurs. Edited by Philip J. Currie and Kevin Padian, 590–593. Cambridge, Massachusetts: Elsevier.
Britt, Brooks B., Peter J. Makovicky, Jacques Gauthier, and Niels Bonde. 1998. “Postcranial Pneumatization in Archaeopteryx.” Nature 395, no. 6700 (September): 374–376.
Brocklehurst, Robert J., Emma R. Schachner, and William I. Sellers. 2018. “Vertebral Morphometrics and Lung Structure in Non-Avian Dinosaurs.” Royal Society Open Science 5, no. 10 (October): 180983.
Brusatte, Stephen L. 2017a. “A Mesozoic Aviary.” Science 355. no. 6327 (24 February)63 27: 792–794.
Brusatte, Stephen. 2017b. “Taking Wing.” Scientific American 316, no. 1 (January): 48–55.
Brusatte, Stephen L., Graeme T. Lloyd, Steve C. Wang, and Mark A. Norell. 2014. “Gradual Assembly of Avian Body Plan Culminated in Rapid Rates of Evolution across the Dinosaur-Bird Transition.” Current Biology 24, no. 20 (20 October): 2386–2392.
Brusatte, Stephen L., Jingmai K. O’Connor, and Erich D. Jarvis. 2015. “The Origin and Diversification of Birds.” Current Biology 25, no. 19 (October 5): R888–R898.
Bryant, Harold N., and Anthony P. Russell. 1993. “The Occurrence of Clavicles within Dinosauria: Implications for the Homology of the Avian Furcula and the Utility of Negative Evidence.” Journal of Vertebrate Paleontology 13, no. 2 (June): 171–184.
Carrano, Matthew T. 2000. “Homoplasy and the Evolution of Dinosaur Locomotion.” Paleobiology 26, no. 3 (Summer): 489–512.
Chatterjee, Sankar, and R. Jack Templin. 2003. “The Flight of Archaeopteryx.” Naturwissenschaften 90, no. 1 (January): 27–32.
Chatterjee, Sankar, and R. Jack Templin. 2012. “Palaeoecology, Aerodynamics, and the Origin of Avian Flight.” In Earth and Life. International Year of Planet Earth. Edited by John A. Talent, 585–612. Berlin, Germany: Springer.
Chen, Pei-ji, Zhi-ming Dong, and Shuo-nan Zhen. 1998. “An Exceptionally Well-Preserved Theropod Dinosaur from the Yixian Formation of China.” Nature 391, no. 6663 (8 January): 147–152.
Chiappe, Luis M. 1995. “The First 85 Million Years of Avian Evolution.” Nature 379, no. 6555 (23 November): 349–355.
Chiappe, Luis M. 2002a. “Osteology of the Flightless Patagopterys deferraraiisi from the Late Cretaceous of Patagonia (Argentina).” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 281–316. Berkeley, California: University California Press.
Chiappe, Luis M. 2002b. “Basal Bird Phylogeny: Problems and Solutions.” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 448–472. Berkeley, California: University California Press.
Chiappe, Luis M. 2009. “Downsized Dinosaurs: The Evolutionary Transition to Modern Birds.” Evolution: Education and Outreach 2, no. 2 (June): 248–256. Special Issue: Transitional Fossils.
Chiappe, Luis M., and Gareth J. Dyke. 2002. “The Mesozoic Radiation of Birds.” Annual Review of Ecology, Evolution and Systematics 33 (November): 91–124.
Chiappe, Luis M., and Gareth J. Dyke. 2006. “The Early Evolutionary History of Birds.” Journal of the Paleontological Society of Korea 22, no. 1: 133–151.
Chiappe, Luis M., Shu’an A. Ji, Qiang Ji, and Mark A. Norell. 1999. “Anatomy and Systematics of the Confuciusornithidae (Theropoda, Aves) from the late Mesozoic of Northeastern China.” Bulletin of the American Museum of Natural History 242 (November): 1–89.
Chiappe, Luis M., Mark A. Norell, and James M. Clark. 1998. “The Skull of a Relative of the Stem-Group Bird Mononykus.” Nature 392, no. 6673 (19 March): 275–278.
Chiappe, Luis M., Mark A. Norell, and James M. Clark. 2002. “The Cretaceous Short-Armed Alvarezsauridae: Mononykus and Its Kin.” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 87–120. Berkeley, California: University California Press.
Choiniere, J.N., X. Xu, J.M. Clark, C.A. Forster, Y. Guo, and F. Han. 2010. “A Basal Alvarezsauroid Theropod from the Early Late Jurassic of Xinjiang, China.” Science 327, no. 5965 (29 January): 571–574.
Choiniere, Jonah N., James M. Clark, Mark A. Norell, and Xing Xu. 2014. “Cranial Osteology of Haplocheirus sollers Choiniere et al., 2010 (Theropoda: Alvarezsauroidea).” American Museum Novitates 3816 (October 22): 1–44.
Christiansen, Per, and Niels Bonde. 2000. “Axial and Appendicular Pneumaticity in Archaeopteryx.” Proceedings of the Royal Society London: Biological Sciences 267, no. 1461 (December 22): 2501–2505.
Cieri, Robert L., Brent A. Craven, Emma R. Schachner, and C. G. Farmer. 2014. “New Insight into the Evolution of the Vertebrate Respiratory System and the Discovery of Unidirectional Airflow in Iguana Lungs.” Proceedings of the National Academy of Sciences 111, no. 48 (November): 17218–17223.
Clark, James M., Teresa Maryañska, and Rinchen Barsbold. 2004. “Therizinosauroidea.” In The Dinosauria. 2nd ed. Edited by David B. Weishampel, Peter Dodson, and Halszka Osmólska, 151–162. Berkeley, California: University of California Press.
Clark, James M., Mark A. Norell, and Luis M. Chiappe. 1999. “An Oviraptorid Skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, Preserved in an Avianlike Brooding Position Over an Oviraptorid Nest.” American Museum Novitates 3265 (May 4):1–36.
Clark, James M., Mark A. Norell, and Peter J. Makovicky. 2002. “Cladistic Approaches to the Relationships of Birds to Other Tetrapod Dinosaurs.” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 31–64. Berkeley, California: University California Press.
Clarke, Julia A., Zhonghe Zhou, and Fucheng Zhang. 2006. “Insight into the Evolution of Avian Flight from a New Clade of Early Cretaceous Ornithurines from China and the Morphology of Yixianornis grabaui.” Journal of Anatomy 208, no. 3 (March): 287–308.
Close, Roger A., and Emily J. Rayfield. 2012. “Functional Morphometric Analysis of the Furcula in Mesozoic Birds.” PLoS ONE 7, no. (May 5 (May 30): e36664. doi:10.1371/journal.pone.0036664.
Codd, Jonathan R., Phillip L. Manning, Mark A. Norell, and Steven F. Perry. 2008. “Avian-like Breathing Mechanics in Maniraptoran Dinosaurs.” Proceedings of the Royal Society London: Biological Sciences 275, no. 1631 (22 January): 157–161.
Currie, Philip J. 1997. “Theropods.” In The Complete Dinosaur. Edited by James O. Farlow, and M. K. Brett-Surman, 216–233. Bloomington, Indiana: Indiana University Press.
Czerkas, Stephen. (no date). “Are Birds Really Dinosaurs?” http://www.dinosaur-museum.org/featheredinosaurs/Are_Birds_Really_Dinosaurs.pdf.
Czerkas, Stephen A., and Alan Feduccia. 2014. “Jurassic Archosaur is a Non-Dinosaurian Bird.” Journal of Ornithology 155, no. 4 (October): 841–851.
Czerkas, Stephen A., and Chongxi Yuan. 2002. “An Arboreal Maniraptoran from Northeast China.” Blanding, Utah: The Dinosaur Museum. http://www.dinosaur-museum.org/featheredinosaurs/arboreal_maniraptoran.pdf.
Dececchi, T. Alexander, and Hans C. E. Larsson. 2013. “Body and Limb Size Dissociation at the Origin of Birds: Uncoupling Allometric Constraints Across a Macroevolutionary Transition.” Evolution 67, no. 9 (September): 2741–2752.
Dececchi, T. Alexander, Hans C. E. Larsson, and Michael B. Habib. 2016. “The Wings Before the Bird: An Evaluation of Flapping-Based Locomotory Hypotheses in Bird Antecedents.” PeerJ 4: e2159. doi:10.7717/peerj.2159.
De Ricqlès, A. J., K. Padian, J. R. Horner, E.-T. Lamm, and N. Myhrvold. 2003. “Osteohistology of Confuciusornis sanctus (Theropoda: Aves).” Journal of Vertebrate Paleontology 23, no. 2 (June 17): 373–386.
Dodson, Peter. 2000. “Origin of Birds: The Final Solution?” American Zoologist 40, no. 4 (August): 504–512.
Dyke, Gareth J., and Mark A. Norell. 2005. “Caudipteryx as a Non-Avian Theropod rather than a Flightless Bird.” Acta Palaeontologica Polonica 50, no. 1: 101–116.
Elzanowski, Andrzej. 2002. “Archaeopterygidae (Upper Jurassic of Germany).” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 129–159. Berkeley, California: University California Press.
Elzanowski, Andrzej, D. Stefan Peters, and Gerald Mayr. 2018. “Cranial Morphology of the Early Cretaceous Bird Confuciusornis.” Journal of Vertebrate Paleontology 38, no. 2 (9 April): e1439832. doi:10.1080/02724634.2018.1439832.
Erickson, Gregory M., Darla K. Zelenitsky, David Ian Kay, and Mark A. Norell. 2017. “Dinosaur Incubation Periods Directly Determined from Growth-Line Counts in Embryonic Teeth Show Reptilian-Grade Development.” Proceedings of the National Academy of Sciences 114, no. 3 (January 3): 540–545.
Fabbri, Matteo, Nicolás Mongiardino Koch, Adam C. Pritchard, Michael Hanson, Eva Hoffman, Gabriel S. Bever, Amy M. Balanoff, et al. 2017. “The Skull Roof Tracks the Brain During the Evolution and Development of Reptiles Including Birds.” Nature Ecology and Evolution 1 (11 September): 1543–1550.
Falk, Amanda R., Thomas G. Kaye, Zhonghe Zhou, and David A. Burnham. 2016. “Laser Fluorescence Illuminates the Soft Tissue and Life Habits of the Early Cretaceous Bird Confuciusornis.” PLoS ONE 11, no. 12 (December 14): e0167284. doi:10.1371/journal.pone.0167284.
Farmer, C. G. 2010. “The Provenance of Alveolar and Parabronchial Lungs: Insights from Paleoecology and the Discovery of Cardiogenic, Unidirectional Airflow in the American Alligator (Alligator mississippiensis).” Physiological and Biochemical Zoology 83, no. 4 (July/August): 561–575.
Farmer, C. G. 2015a. “The Evolution of Unidirectional Pulmonary Airflow.” Physiology 30, no. 4 (July): 260–272.
Farmer, C. G. 2015b. “Similarity of Crocodilian and Avian Lungs Indicates Unidirectional Flow is Ancestral for Archosaurs.” Integrative and Comparative Biology 55, no. 56 (December): 1–10.
Farmer, C. G., and Kent Sanders. 2010. “Unidirectional Airflow in the Lungs of Alligators.” Science 327, no. 5963 (15 January): 338–340.
Feduccia, Alan. 1999a. The Origin and Evolution of Birds. 2nd ed. New Haven, Connecticut: Yale University Press.
Feduccia, Alan. 1999b. “1,2,3 = 2,3,4: Accommodating the Cladogram.” Proceedings of the National Academy of Sciences 96, no. 9 (April 27): 4740–4742.
Feduccia, A. 2001. “The Problem of Bird Origins and Early Avian Evolution.” Journal of Ornithology 142, Supplement 1 (June): 139–147.
Feduccia, Alan. 2012. Riddle of the Feathered Dragons. Hidden Birds of China. New Haven, Connecticut: Yale University Press.
Feduccia, Alan, and Stephen A. Czerkas. 2015. “Testing the Neoflightless Hypothesis: Propatagium Reveals Flying Ancestry of Oviraptorosaurs.” Journal of Ornithology 156 (19 March): 1067–1074.
Feduccia, Alan, and Larry D. Martin. 1998. “Theropod-bird Link Reconsidered.” Nature 391, no. 6669 (19 February):754.
Feduccia, A., and R. Wild. 1993. “Birdlike Characters in the Triassic Archosaur Megalancosaurus.” Naturwissenschaften 80 (December): 564–566.
Forster, Catherine A., Scott D. Sampson, Luis M. Chiappe, and David W. Krause. 1998. “The Theropod Ancestry of Birds: New Evidence from the Late Cretaceous of Madagascar.” Science 279, no. 5358 (March): 1915–1919.
Foth, Christian, Oliver W. M. Rauhut, and Helmut Tischlinger. 2015. “Als die Federn Lliegen Lernten.” Spektrum der Wissenschaft 4/2015 (April): 28–33.
Foth, Christian, and Oliver W. M. Rauhut. 2017. “Re-evaluation of the Haarlem Archaeopteryx and the Radiation of Maniraptoran Theropod Dinosaurs.” BMC Evolutionary Biology 17, (2 December). doi:10.1186/s12862-017-1076-y.
Foth, Christian, Helmut Tischlinger, and Oliver W. M. Rauhut. 2014. “New Specimen of Archaeopteryx Provides Insights into the Evolution of Pennaceous Feathers.” Nature 511 (2 July): 79–82.
Funston, Gregory F., Philip J. Currie, David A. Eberth, Michael J. Ryan, Tsogtbaatar Chinzorig, Demchig Badamgarav, and Nicholas R. Longrich. 2016. “The First Oviraptorosaur (Dinosauria: Theropoda) Bonebed: Evidence of Gregarious Behavior in a Maniraptoran Theropod.” Scientific Reports 6 (October 21): 35782. doi:10.1038/srep35782.
Gatesy, Stephen M., and Kenneth P. Dial. 1996. “From Frond to Fan: Archaeopteryx and the Evolution of Short-Tailed Birds.” Evolution 50, no. 5 (October): 2037–2048.
Geist, Nicholas R., and Alan Feduccia. 2000. “Gravity-Defying Behaviors: Identifying Models for Protoaves.” American Zoologist 40, no. 4 (August): 664–675.
Glaubrecht, Mathias. 1998. “Archys Sippschaft.” Bild der Wissenschaft 9: 32–37.
Grellet-Tinner, Gerald, and Luis M. Chiappe. 2004. “Dinosaur Eggs and Nesting: Implications for Understanding the Origin of Birds.” In Feathered Dinosaurs: Studies on the Transition from Dinosaurs to Birds. Edited by Philip J. Currie, Eva B. Koppelhus, Martin A. Shugar, and Joanna L. Wright, 185–214. Bloomington, Indiana: Indiana University Press.
Grellet-Tinner, Gerald, and Peter Makovicky. 2006. “A Possible Egg of the Dromaeosaur Deinonychus antirrhopus: Phylogenetic and Biological Implications.” Canadian Journal of Earth Sciences 43, no. 6 (June): 705–719.
Hall, Brian K., and Matthew K. Vickaryous. 2015. “Merrythoughts of the Past and Present: Revisiting the Homology of the Furcula.” In All Animals are Interesting: A Festschrift in Honour of Anthony P. Russell. Edited by O. R. P. Bininda-Emonds, G. L. Powell, H. A. Jamniczky, A. M. Bauer, and J. Theodor, 439–454. All Animals are Interesting: A Festschrift in Honour of Anthony P. Russell. Oldenburg, Germany: BIS Verlag.
Heilmann, Gerhard. 1926. The Origin of Birds. London, United Kingdom: Witherby.
Hejnol, Andreas. 2014. “Excitation Over Jelly Nerves.” Nature 510, no. 7503 (21 May): 38–39.
Hendrickx, Christophe, Scott A. Hartman, and Octávio Mateus. 2015. “An Overview of Non-Avian Theropod Discoveries and Classification.” PalArch’s Journal of Vertebrate Palaeontology 12, no. 1 :1–73.
Hincke, Maxwell T., Yves Nys, Joel Gautron, Karlheinz Mann, Alejandro B. Rodriguez-Navarro, and Marco D. McKee. 2012. “The Eggshell: Structure, Composition and Mineralization.” Frontiers in Bioscience 17, no. 4 (January 1): 1266–1280.
Holtz, Thomas R. Jr. 1998. “A New Phylogeny of the Carnivorous Dinosaurs.” Gaia 15 (December): 5–61.
Holtz, Thomas R. Jr. 1994. “The Phylogenetic Position of the Tyrannosauridae: Implications for Theropod Systematics.” Journal of Paleontology 68, no. 5 (September): 1100–1117.
Holtz, Thomas R. Jr. 2001. “Arctometatarsalia Revisited: The Problem of Homoplasy in Reconstructing Theropod Phylogeny.” In New Perspectives on the Origin and Early Evolution of Birds. Edited by Jacques Gauthier, and Lawrence F. Gall, 99–121. New Haven, Connecticut: Peabody Museum of Natural History.
Horner, John R., and Robert Makela. 1979. “Nest of Juveniles Provides Evidence of Family Structure Among Dinosaurs.” Nature 282, no. 5736 (15 November): 296–298.
Hou, Lianhai. 2001. Mesozoic Birds of China. Institute of Vertebrate Paleontology and Paleoanthropology. Trans. Will Downs. Taiwan, China: Phoenix Valley Provincial Aviary of Taiwan.
Hou, Lianhai, Larry D. Martin, Zhonghe Zhou, and Alan Feduccia. 1996. “Early Adaptive Radiation of Birds: Evidence from Fossils from Northeastern China.” Science 274, no. 5290 (15 November): 1164–1167.
Hou, Lianhai, Larry D. Martin, Zhonghe Zhou, Alan Feduccia, and Fucheng Zhang. 1999. “A Diapsid Skull in a New Species of the Primitive Bird Confuciusornis.” Nature 399, no. 6737 (17 June): 679–682.
Hu, Han, Jingmai K. O’Connor, and Zhonghe Zhou. 2015. “A New Species of Pengornithidae (Aves: Enantiornithes) from the Lower Cretaceous of China Suggests a Specialized Scansorial Habitat Previously Unknown in Early Birds.” PLoS ONE 10, no. 6 (June 3): e0126791. doi:10.1371/journal.pone.0126791.
Jackson, Frankie D., John R. Horner, and David J. Varricchio. 2010. “A Study of a Troodon Egg Containing Embryonic Remains Using Epifluorescence Microscopy and Other Techniques.” Cretaceous Research 31, no. 2 (April): 255–262.
Jenkins, Farish A. Jr., Kenneth P. Dial, and G. E. Goslow Jr. 1988. “A Cineradiographic Analysis of Bird Flight: The Wishbone in Starlings is a Spring.” Science 241, no. 4872 (16 September): 1495–1498.
Jones, Terry D., James O. Farlow, John A. Ruben, Donald M. Henderson, and Willem J. Hillenius. 2000. “Cursoriality in Bipedal Archosaurs.” Nature 406, no. 6797 (17 August): 716–718.
Jones, Terry D., and John A. Ruben. 2001. “Respiratory Structure and Function in Theropod Dinosaurs and Some Related Taxa.” In New Perspectives on the Origin and Early Evolution of Birds. Edited by Jacques Gauthier, and Lawrence F. Gall, 443–461. New Haven, Connecticut: Peabody Museum of Natural History.
Junker, Reinhard. 2015. “Alte Vögel mit moderner Flugkunst.” https://www.genesisnet.info/schoepfung_evolution/n233.php.
Junker, Reinhard. 2017. “Dino-Federvieh: Zum Ursprung von Vogelfeder und Vogelflug.” W+W Special Paper B-17-1, November. https://www.wort-und-wissen.org/wp-content/uploads/b-17-1_feder-und-flug.pdf.
Junker, Reinhard. 2018a. “Neuartiger Federtyp bei mutmaßlichem Dinosaurier.” https://www.genesisnet.info/schoepfung_evolution/n257.php.
Junker, Reinhard. 2018b. “Serikornis—Dinosaurier mit Halbfertigen Federn?” Studium Integrale Journal 24 (May): 44–47.
Kämpfe, L. 2003. “Federentstehung und Vogelflug—Neue Evolutionsbiologische Gesichtspunkte.“ Praxis der Naturwissenschaften—Biologie 6, no. 52 (September): 40–48.
Kaplan, Matt. 2013. “Theory Suggests Iconic Early Bird Lost Its Flight.” Nature (12 November). doi:10.1038/nature.2013.14142.
Kundrát, Martin. 2007. “Avian-like Attributes of a Virtual Brain Model of the Oviraptorid Theropod Conchoraptor gracilis.” Naturwissenschaften 94, no. 6 (June): 499–504.
Kundrát, Martin, Arthur R. I. Cruickshank, Terry W. Manning, and John Nudds. 2008. “Embryos of Therizinosauroid Theropods from the Upper Cretaceous of China: Diagnosis and Analysis of Ossification Patterns.” Acta Zoologica 89, no. 3 (July): 231–251.
Kurochkin, Evgeny N. 1985. “A True Carinate Bird From Lower Cretaceous Deposits in Mongolia and Other Evidence of Early Cretaceous Birds in Asia.” Cretaceous Research 6, no. 3 (September): 271–278.
Kurochkin, Evgeny N. 1999. “The Relationships of the Early Cretaceous Ambiortus and Otogornis (Aves: Ambiortiformes).” In Avian Paleontology at the Close of the 20th Century: Proceedings of the 4th International Meeting of the Society of Avian Paleontology and Evolution. Edited by Storrs L. Olson, 275–284. Smithsonian Contributions to Paleobiology 89.
Kurochkin, E. N. 2006. “Parallel Evolution of Theropod Dinosaurs and Birds.” Entomological Review 86 (January): S45–S58.
Lautenschlager, Stephan, Lawrence M. Witmer, Perle Altangerel, and Emily J. Rayfield. 2013 “Edentulism, Beaks, and Biomechanical Innovations in the Evolution of Theropod Dinosaurs.” Proceedings of the National Academy of Sciences 110, no. 51 (December 2): 20657–20662.
Lee, Sang-im, Jooha Kim, Hyungmin Park, Piotr G. Jabloński, and Haecheon Choi. 2015. “The Function of the Alula in Avian Flight.” Scientific Reports 5 (7 May): 9914.
Lefèvre, Ulysse, Andrea Cau, Aude Cincotta, Dongyu Hu, Anusuya Chinsamy, François Escuillié, and Pascal Godefroit. 2017. “A New Jurassic Theropod from China Documents a Transitional Step in the Macrostructure of Feathers.” The Science of Nature 104 (22 August): 74.
Leigh, Egbert Giles Jr. 2014. “Alan Feduccia’s Riddle of the Feathered Dragons: What Reptiles Gave Rise to Birds?” Evolution: Education and Outreach 7: 9.
LeMaster, James C. 2018. “Evolution’s Waiting-Time Problem and Suggested Ways to Overcome It—A Critical Survey.” BIO-Complexity 2018, no. 2 (July 17): 1–9. doi:10.5048/BIO-C.2018.2.
Lipkin, Christine, Paul C. Sereno, and John R. Horner. 2007. “The Furcula in Suchomimus tenerensis and Tyrannosaurus rex (Dinosauria: Theropoda: Tetanurae).” Journal of Paleontology 81, no. 6 (November): 1523–1527.
Liu, Di, L. M. Chiappe, Yuguang Zhang, F. J. Serrano, and Qingjin Meng. 2018. “Soft Tissue Preservation in Two New Enantiornithine Specimens (Aves) From the Lower Cretaceous Huajiying Formation of Hebei Province, China.” Cretaceous Research 95 (March): 191–207.
Louchart, Antoine, and Laurent Viriot. 2011. “From Snout to Beak: The Loss of Teeth in Birds.” Trends in Ecology and Evolution 26, no. 12 (December): 663–673.
Makovicky, Peter J., Sebastián Apesteguía, and Federico L. Agnolín. 2005. “The Earliest Dromaeosaurid Theropod from South America.” Nature 437, no. 7061 (13 October): 1007–1011.
Makovicky, Peter J., and Philip J. Currie. 1998. “The Presence of a Furcula in Tyrannosaurid Theropods, and Its Phylogenetic and Functional Implications.” Journal of Vertebrate Paleontology 18, no. 1 (April 10): 143–149.
Makovicky, Peter J., and Mark A. Norell. 2004. “Troodontidae.” In The Dinosauria. 2nd ed. Edited by David B. Weishampel, Peter Dodson, and Halszka Osmólska, 184–195. Berkeley, University of California Press.
Makovicky, Peter J., and Lindsay E. Zanno. 2011. “Theropod Diversity and the Refinement of Avian Characteristics.” In Living Dinosaurs: The Evolutionary History of Modern Birds. Edited by Gareth Dyke, and Gary Kaiser, 9–29. Oxford, United Kingdom: John Wiley and Sons.
Martin, Larry D. 1985. “The Relationship of Archaeopteryx to Other Birds.” In The Beginnings of Birds: Proceedings of the International Archaeopteryx Conference. Edited by Max K. Hecht, John H. Ostrom, Günter Viohl, and Peter Wellnhofer, 177–183. Eichstätt, Germany: Friends of the Jura Museum.
Martin, L. D. 2004. “A Basal Archosaurian Origin For Birds.” Acta Zoologica Sinica 50, no. 6 (1 January): 978–990.
Martin, Larry D., and Stephan A. Czerkas. 2000. “The Fossil Record of Feather Evolution in the Mesozoic.” American Zoologist 40, no. 4 (August): 687–694.
Martin, Larry D., and Zhonghe Zhou. 1997. “Archaeopteryx-Like Skull in Enantiornithine Bird.” Nature 389, no. 6651 (9 October): 556.
Martin, L. D., Z. Zhou., L. Hou, and A. Feduccia. 1998. “Confuciusornis sanctus Compared to Archaeopteryx lithographica.” Naturwissenschaften 85 (2 March): 286–289.
Martyniuk, Matthew P. 2012. A Field Guide to Mesozoic Birds and Other Winged Dinosaurs. Vernon, New Jersey: Pan Aves.
Mayr, Gerald. 2017a. Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance. Chichester, United Kingdom: Wiley.
Mayr, Gerald. 2017b. “Pectoral Girdle Morphology of Mesozoic Birds and the Evolution of the Avian Supracoracoideus Muscle.” Journal of Ornithology 158 (23 March): 859–867.
Maryañska, Teresa, Halszka Osmólska, and Mieczysław Wolsan. 2002. “Avialan Status for Oviraptorosauria.” Acta Palaeontologica Polonica 47, no. 1: 97–116.
Meng, Qingjin, Jinyuan Liu, David J. Varricchio, Timothy Huang, and Chunling Gao. 2004. “Parental Care in an Ornithischian Dinosaur.” Nature 431 (8 September): 145–146.
Meredith, Robert W., Guojie Zhang, M. Thomas P. Gilbert, Erich D. Jarvis, and Mark S. Springer. 2015. “Evidence For a Single Loss of Mineralized Teeth in the Common Avian Ancestor.” Science 346, no. 5215 (12 December): 1336.
Molnar, Ralph E. 1985. “Alternatives to Archaeopteryx: A Survey of Proposed Early or Ancestral Birds.” In The Beginnings of Birds: Proceedings of the International Archaeopteryx Conference. Edited by Max K. Hecht, John H. Ostrom, Günter Viohl, and Peter Wellnhofer, 209–217. Eichstätt, Germany: Friends of the Jura Museum.
Moroz, Leonid L.. Kevin M. Kocot, Mathew R. Citarella, Sohn Dosung, Tigran P. Horekian, Inna S. Povolotskaya, Anastasia P. Grigorenko, et al. 2014. “The Ctenophore Genome and the Evolutionary Origins of Neural Systems.” Nature 510, no. 7503 (21 May): 109–144.
Moser, Markus. 2014. “Federkleid von Archaeopteryx und die Evolution des Fluges.” Naturwissenschaftliche Rundschau 67, no. 794: 416–417.
Naish, Darren. 2014. “50 Million Years of Incredible Shrinking Theropod Dinosaurs.” July 31. https://blogs.scientificamerican.com/tetrapod-zoology/50-million-years-of-incredible-shrinking-theropod-dinosaurs/.
Navalón, Guillermo, Jesús Marugán-Lobón, Luis M. Chiappe, José Luis Sanz, and Ángela D. Buscalioni. 2015. “Soft-Tissue and Dermal Arrangement in the Wing of an Early Cretaceous Bird: Implications For the Evolution of Avian Flight.” Scientific Reports 5 (October 6): 1–7.
Nesbitt, Sterling J., Alan H. Turner, Michelle Spaulding, Jack L. Conrad, and Mark A. Norell. 2009. “The Theropod Furcula.” Journal of Morphology 270, no. 7 (July): 856–879.
Norell, Mark A., Amy M. Balanoff, Daniel E. Barta, and Gregory M. Erickson. 2018 “A Second Specimen of Citipati Osmolskae Associated With a Nest of Eggs from Ukhaa Tolgod, Omnogov Aimag, Mongolia.” American Museum Novitates 3899 (26 April): 1–44.
Norell, M. A., L. Chiappe, and J. M. Clark. 1993. “Mononykus olecranus, An Unusual New Bird From the Cretaceous of Mongolia.” Journal of Vertebrate Paleontology 13:51A. Supplement 3.
Norell, Mark A., James M. Clark, Luis M. Chiappe, and Demberelyin Dashzeveg. 1995. “A Nesting Dinosaur.” Nature 378, no. 6559 (28 December): 774–776.
Norell, Mark A., James M. Clark, Alan H. Turner, Peter J. Makovicky, Rinchen Barsbold, and Timothy Rowe. 2006. “A New Dromaeosaurid Theropod From Ukhaa Tolgod (Ömnögov, Mongolia).” American Museum Novitates 3545 (7 December): 1–51.
Norell, Mark A., and Peter J. Makovicky. 1999. “Important Features of the Dromaeosaurid Skeleton. II: Information From Newly Collected Specimens of Velociraptor mongoliensis.” American Museum Novitates 3282 (December 8): 1–45.
Norell, Mark A., and Peter J. Makovicky. 2004. “Dromaeosauridae.” In The Dinosauria. 2nd ed. Edited by David B. Weishampel, Peter Dodson, and Halszka Osmólska, 184–209. Berkeley, California: University of California Press.
Norell, Mark A., Peter Makovicky, and James A. Clark. 1997. “A Velociraptor Wishbone.” Nature 389, no. 6650 (2 October): 447.
Norman, D. B. 1990. “Problematic Theropoda: ‘Coelurosaurs’.” In The Dinosauria. Edited by David B. Weishampel, Peter Dodson, and Halszka Osmólska, 280–305. Berkeley, California: University of California Press.
Novas, Fernando E., and Diego Pol. 2002. “Alvarezsaurid Relationships Reconsidered.” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 121–128. Berkeley, California: University California Press.
Novas, Fernando E., and Pablo F. Puerta. 1997. “New Evidence Concerning Avian Origins From the Late Cretaceous of Patagonia.” Nature 387, no. 6631 (22 May): 390–392.
O’Connor, Jingmai K. 2019. “The Trophic Habits of Early Birds.” Palaeogeography, Palaeoclimatology, Palaeoecology 513 (1 January): 178–195.
O’Connor, Jingmai K., Luis M. Chiappe, and Alyssa Bell. 2011. “Pre-Modern Birds: Avian Divergences in the Mesozoic.” In Living Dinosaurs: The Evolutionary History of Modern Birds. Edited by Gareth Dyke, and Gary Kaiser, 39–114. Oxford, United Kingdom: John Wiley and Sons.
O’Connor, Jingmai K., Ke-Qin Gao, and Luis M. Chiappe. 2010. “A New Ornithuromorph (Aves: Ornithothoraces) Bird From the Jehol Group Indicative of Higher-Level Diversity.” Journal of Vertebrate Paleontology 30, no. 2 (24 March): 311–321.
O’Connor, Jingmai K., and Corwin Sullivan. 2014. “Reinterpretation of the Early Cretaceous Maniraptoran (Dinosauria: Theropoda) Zhongornis haoae as a Scansoriopterygid-like Non-avian, and Morphological Resemblances Between Scansoriopterygids and Basal Oviraptorosaurs.” Vertebrata PalAsiatica 52 (January): 3–30.
O’Connor, Jingmai K., Chengkai Sun, Xing Xu, Xiaolin Wang, and Zhonghe Zhou. 2012. “A New Species of Jeholornis With Complete Caudal Integument.” Historical Biology 24, no. 1 (29 November): 29–41.
O’Connor, Jingmai K., Xiaoli Wang, Corwin Sullivan, Xiaoting Zheng, Pablo Tubaro, Xiaomei Zhang, and Zhonghe Zhou. 2013. “Unique Caudal Plumage of Jeholornis and Complex Tail Evolution in Early Birds.” Proceedings of the National Academy of Sciences 110, no. 43 (September 12): 17404–17408.
O’Connor, Jingmai K., Xiaoli Wang, Xiaoting Zheng, Han Hu, Xiaomei Zhang, and Zhonghe Zhou. 2015a. “An Enantiornithine With a Fan-Shaped Tail, and the Evolution of the Rectricial Complex in Early Birds.” Current Biology 26, no. 1 (11 January): 114–119.
O’Connor, J. K., and N. V. Zelenkov. 2013. “The Phylogenetic Position of Ambiortus: Comparison With Other Mesozoic Birds from Asia.” Paleontological Journal 47 (19 December): 1270–1281.
O’Connor, Jingmai K., Xiao-Ting Zheng, Han Hu, Xiao-Li Wang, and Zhong-He Zhou. 2017. “The Morphology of Chiappeavis magnapremaxillo (Pengornithidae: Enantiornithes) and a Comparison of Aerodynamic Function in Early Cretaceous Avian Tail Fans.” Vertebrata PalAsiatica 55, no. 4: 41–58.
O’Connor, Jingmai K., Xiaoting Zheng, Corwin Sullivan, Cheng-Ming Chuong, Xiaoli Wang, Ang Li, Yan Wang, Xiaomei Zhang, and Zhonghe Zhou. 2015b. “Evolution and Functional Significance of Derived Sternal Ossification Patterns in Ornithothoracine Birds.” Journal of Evolutionary Biology 28, no. 8 (June): 1550–1567.
O’Connor, Jingmai K., Xiaoting Zheng, Xiaoli Wang, Yan Wang, and Zhonghe Zhou. 2014. “Ovarian Follicles Shed New Light on Dinosaur Reproduction During the Transition Towards Birds.” National Science Review 1, no. 1 (March): 15–17.
O’Connor, Jingmai K., Xiaoting Zheng, Xiaoli Wang, Xiao-Mei Zhang, and Zhong-He Zhou. 2015c. “The Gastral Basket in Basal Birds and Their Close Relatives: Size and Possible Function.” Vertebrata PalAsiatica 53, no. 2:133–152.
O’Connor, Jingmai K., and Zhonghe Zhou. 2013. “A Redescription of Chaoyangia beishanensis (Aves) and a Comprehensive Phylogeny of Mesozoic Birds.” Journal of Systematic Palaeontology 11, no. 7 (January): 889–906.
O’Connor, Jingmai K., and Zhonghe Zhou. 2015. “Early Evolution of the Biological Bird: Perspectives From New Fossil Discoveries in China.” Journal of Ornithology 156 (21 April), Supplement 1: 333–342.
O’Connor, Patrick M., and Leon P. A. M. Claessens. 2005. “Basic Avian Pulmonary Design and Flow-Through Ventilation in Non-Avian Theropod Dinosaurs.” Nature 436, no. 7048 (14 July): 253–256.
Olson, Storrs L. 2002. “Review: ‘New Perspectives on the Origin and Early Evolution of Birds. Proceedings of the International Symposium in Honor of John H. Ostrom.’” The Auk 119, no. 4 (1 October): 1202–1205.
Olson, Storrs L., and Alan Feduccia. 1979. “Flight Capability and the Pectoral Girdle of Archaeopteryx.” Nature 278, no. 5701 (March 15): 247–248.
Organ, Chris L., Andrew M. Shedlock, Andrew Meade, Mark Pagel, and Scott V. Edwards. 2007. “Origin of Avian Genome Size and Structure in Non-Avian Dinosaurs.” Nature 446, no. 7132 (8 March): 180–184.
Padian, Kevin, and Luis M. Chiappe. 1998a. “The Origin of Birds and Their Flight.” Scientific American 278, no. 2 (February): 38–47.
Padian, Kevin, and Luis M. Chiappe. 1998b. “The Origin and Early Evolution of Birds.” Biological Reviews 73, no. 1 (February): 1–42.
Paul, Gregory S. 2001. “Were the Respiratory Complexes of Predatory Dinosaurs Like Crocodilians or Birds?” In New Perspectives on the Origin and Early Evolution of Birds.Edited by J. Gauthier, and L. F. Gall, 460–482. New Perspectives on the Origin and Early Evolution of Birds. New Haven, Connecticut: Peabody Museum of Natural History.
Persons, W. Scott IV, Philip J. Currie, and Mark A. Norell. 2014. “Oviraptorosaur Tail Forms and Functions.” Acta Palaeontologica Polonica 59, no. 3 (September): 553–567.
Peters, D. S. 1985. “Functional and Constructive Limitations in the Early Evolution of Birds.” In The Beginnings of Birds: Proceedings of the International Archaeopteryx Conference. Edited by Max K. Hecht, John H. Ostrom, Günter Viohl, and Peter Wellnhofer, 243–249. Eichstätt, Germany: Friends of the Jura Museum.
Peters D. S. 1994. “The Origin of Birds. Do Recent Fossil Discoveries Change the Model?” In Morphology and Evolution. Symposia on the Occasion of the 175th Anniversary of the Senckenbergische Naturforschende Gesellschaft. Edited by Dieter Mollenhauer, D. S. Peters, and Wolfgang F. Gutmann, 403–423. Frankfurt, Germany: Verlag Waldemar Kramer.
Peters, Dieter Stefan. 2002. “Anagenesis of Early Birds Reconsidered.” Senckenbergiana lethaea 82 (June): 347–354.
Peters, D. S., and W. F. Gutmann. 1976. “The Position of the ‘Primitive Bird’ Archaeopteryx in the Derivation Model of Birds.” Nature and Museum 106: 265–275.
Peters, Dieter Stefan, and Qiang Ji. 1999. “Did Confuciusornis Have to Climb?” Journal of Ornithology 140 (1 January): 41–50.
Pickrell, John. 2017. “Dinosaur’s Feathers Cast in New Light.” Nature 551, no. 7678 (2 November): 17.
Proctor, Noble S., and Patrick J. Lynch. 1993. Manual of Ornithology: Avian Structure and Function. New Haven, Connecticut: Yale University Press.
Prum, Richard O. 2008. “Who’s Your Daddy?” Science 322, no. 5909 (January): 1799–1800.
Prum, Richard O. 2010. “Moulting Tail Feathers in a Juvenile Oviraptorisaur.” Nature 468, no. 7320 (4 November): E1.
Purves, William K., David E. Sadava, Gordon H. Orians, and H. Craig Heller. 2003. Life: The Science of Biology. 7th ed. Sunderland, Massachusetts: Sinauer Associates.
Puttick, Mark N., Gavin H. Thomas, Michael J. Benton, and P. David Polly. 2014. “High Rates of Evolution Preceded the Origin of Birds.” Evolution 68, no. 5 (February): 1497–1510.
Quick, Devon E., and John A. Ruben. 2009. “Cardio-Pulmonary Anatomy in Theropod Dinosaurs: Implications From Extant Archosaurs.” Journal of Morphology 270, no. 10 (October): 1232–1246.
Rashid, Dana J., Susan C. Chapman, Hans C. E. Larsson, Chris L. Organ, Anne-Gaelle Bebin, Christa S. Merzdorf, Roger Bradley, and John R. Horner. 2014. “From Dinosaurs to Birds: A Tail of Evolution.” Evo-Devo 5: 25. https://doi.org/10.1186/2041-9139-5-25.
Rashid, Dana J., Kevin Surya, Luis M. Chiappe, Nathan Carroll, Kimball L. Garrett, Bino Varghese, Alida Bailleul, Jingmai K. O’Connor, Susan C. Chapman, and John R. Horner. 2018. “Avian Tail Ontogeny, Pygostyle Formation, and Interpretation of Juvenile Mesozoic Specimens.” Scientific Reports 8 (13 June): 9014.
Rauhut, Oliver W. M., Christian Foth, and Helmut Tischlinger. 2018. “The Oldest Archaeopteryx (Theropoda: Avialae): A New Specimen from the Kimmeridgian/Tithonian Boundary of Schamhaupten, Bavaria.” PeerJ 6 (January): e4191. doi: 10.7717/peerj.4191
Rayner, J. M. V. 2001. “On the Origin and Evolution of Flapping Flight Aerodynamics in Birds.” In New Perspectives on the Origin and Early Evolution of Birds. Edited by J. Gauthier, and L. F. Gall, 363–383. New Perspectives on the Origin and Early Evolution of Birds. New Haven, Connecticut: Peabody Museum of Natural History.
Ruben, John A., and Terry D. Jones. 2000. “Selective Factors Associated With the Origin of Fur and Feathers.” American Zoologist 40, no. 4 (August): 585–596.
Ruben, John A., Terry D. Jones, Nicholas R. Geist, and W. Jaap Hillenius. 1997. “Lung Structure and Ventilation in Theropod Dinosaurs and Early Birds.” Science 278, no. 5341 (November 14): 1267–1270.
Ruben, John A., Cristiano Dal Sasso, Nicholas R. Geist, Willem J. Hillenius, Terry D. Jones, and Marco Signore. 1999. “Pulmonary Function and Metabolic Physiology of Theropod Dinosaurs.” Science 283, no. 5401 (February): 514–516.
Saitta, Evan Thomas, Rebecca Gelernter, and Jakob Vinther. 2017. “Additional Information on the Primitive Contour and Wing Feathering of Paravian Dinosaurs.” Palaeontology 61, no. 4 (November): 1–16.
Sanz, José L., Luis M. Chiappe, Bernardino P. Pérez-Moreno, Angela D. Buscalioni, José J. Moratalla, Francisco Ortega, and Francisco J. Poyato-Ariza. 1996. “An Early Cretaceous Bird From Spain and Its Implications For the Evolution of Avian Flight.” Nature 382, no. 6590 (1 August): 442–445.
Sanz, José L., Bernardino P. Pérez-Moreno, Luis M. Chiappe, and Angela D. Buscalioni. 2002. “The Birds from the Lower Cretaceous of Las Hoyas (Province of Cuenca, Spain).” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 209–229. Berkeley, California: University of California Press.
Sato, Tamaki, Yen-Nien Cheng, Xiao-Chun Wu, Darla K. Zelenitsky, and Yu-Fu Hsiao. 2005. “A Pair of Shelled Eggs Inside a Female Dinosaur.” Science 308, no. 5720 (15 April): 375.
Schachner, Emma R., Robert L. Cieri, James P. Butler, and C. G. Farmer. 2014. “Unidirectional Pulmonary Airflow Patterns in the Savannah Monitor Lizard.” Nature 506, no. 7489 (11 December): 367–371.
Schachner, Emma R., C. G. Farmer, Andrew T. McDonald, and Peter Dodson. 2011. “Evolution of the Dinosauriform Respiratory Apparatus: New Evidence From the Postcranial Axial Skeleton.” The Anatomical Record 294, no. 9 (September): 1532–1547.
Schachner, Emma R., John R. Hutchinson, and C. G. Farmer. 2013. “Pulmonary Anatomy in the Nile Crocodile and the Evolution of Unidirectional Airflow in Archosauria.” PeerJ 1 (March): e60. https://doi.org/10.7717/peerj.60.
Scherer, Siegfried. ed. 1993. Typen des Lebens. Berlin, Germany: Studium Integrale.
Schmidt-Nielsen, Knut. 1971. “How Birds Breathe.” Scientific American 225, no. 6 (December 1): 72–79.
Senter, Phil. 2005. “Function in the Stunted Forelimbs of Mononykus olecranus (Theropoda), a Dinosaurian Anteater.” Paleobiology 31, no. 3 (Summer): 373–381.
Sereno, Paul C. 1997. “The Origin and Evolution of Dinosaurs.” Annual Reviews of Earth and Planetary Sciences 25: 435–489.
Sereno, Paul C. 1999. “The Evolution of Dinosaurs.” Science 284, no. 5423 (June 25): 2137–2147.
Sereno, Paul C. 2004. “Birds as Dinosaurs.” Acta Zoologica Sinica 50, no. 6 (January): 991–1001.
Sereno, Paul C., Ricardo N. Martinez, Jeffrey A. Wilson, David J. Varricchio, Oscar A. Alcober, and Hans C. E. Larsson. 2008. “Evidence for Avian Intrathoracic Air Sacs in a New Predatory Dinosaur From Argentina.” PLoS ONE 3, no. 9 (September 30): e3303. doi: 10.1371/journal.pone.0003303.
Shipman, Pat. 1997. “Birds Do It . . . Did Dinosaurs?” New Scientist 2067 (1 February): 27–31.
Shipman, Pat. 1998. Taking Wing: Archaeopteryx and the Evolution of Bird Flight. New York, New York: Simon and Schuster.
Stokstad, Erik. 2002. “Fossil Bird From China Turns Tail, Spills Guts.” Science 297, no. 5581 (26 July): 495–496.
Sullivan, Corwin, David W. E. Hone, Xing Xu, and Fucheng Zhang. 2010. “The Asymmetry of the Carpal Joint and the Evolution of Wing Folding in Maniraptoran Theropod Dinosaurs.” Proceedings of the Royal Society B 277, no. 1690 (7 July): 2027–2033.
Sullivan, Corwin, Yuan Wang, David W. E. Hone, Yuanqing Wang, Xing Xu, and Fucheng Zhang. 2014. “The Vertebrates of the Jurassic Daohugou Biota of Northeastern China.” Journal of Vertebrate Paleontology 34, no. 2 (March): 243–280.
Sullivan, Corwin, Xing Xu, and Jingmai K. O’Connor. 2017. “Complexities and Novelties in the Early Evolution of Avian Flight, As Seen in the Mesozoic Yanliao and Jehol Biotas of Northeast China.” Palaeoworld 26, no. 2 (June): 212–229.
Sumida, Stuart S., and Christopher A. Brochu. 2000. “Phylogenetic Context For the Origin of Feathers.” American Zoologist 40, no. 4 (August): 486–503.
Thomas, A. L. R., and J. P. Garner. 1998. “Are Birds Dinosaurs?” Trends in Ecology and Evolution 13, no. 4 (April 1): 129–130.
Tickle, P. G., M. A. Norell, and J. R. Codd. 2012. “Ventilatory Mechanics From Maniraptoran Theropods to Extant Birds.” Journal of Evolutionary Biology 25, no. 4 (April): 740–747.
Tsuihiji, Takanobu, Lawrence M. Witmer, Mahito Watabe, Rinchen Barsbold, Khishigjav Tsogtbaatar, Shigeru Suzuki, and Purevdorj Khatanbaatar. 2017. “New Information On the Cranial Morphology of Avimimus (Theropoda: Oviraptorosauria).” Journal of Vertebrate Paleontology 37, no. 4 (29 August). doi: 10.1080/02724634.2017.1347177.
Turner, Alan H., Peter J. Makovicky, and Mark A. Norell. 2012. “A Review of Dromaeosaurid Systematics and Paravian Phylogeny.” Bulletin of the American Museum, Natural History 371 (August): 1–206.
Tykoski, Ronald S., Catherine A. Forster, Timothy Rowe, Scott D. Sampson, and Darlington Munyikwa. 2002. “A Furcula in the Coelophysid Theropod Syntarsus.” Journal of Vertebrate Paleontology 22,no. 3 (September 19): 728–733.
Ullrich, Henrik. 2008. “Sind Vogelflügel umgestaltete Dinosaurierhände? Zum Konflikt zwischen fossilen und entwicklungsbiologischen Daten bei der phylogenetischen Herleitung des Vogelflügels.” Studium Integrale Journal 15, no. 1 (April): 18–30.
Varricchio, David J., Frankie Jackson, John J. Borkowski, and John R. Horner. 1997. “Nest and Egg Clutches of the Dinosaur Troodon formosus and the Evolution of Avian Reproductive Traits.” Nature 385, no. 6613 (16 January): 247–250.
Varricchio, David J., and Frankie D. Jackson. 2004a. “Two Eggs Sunny-Side Up: Reproductive Physiology in the Dinosaur Troodon formosus.” In Feathered Dragons: Studies on the Transition From Dinosaurs to Birds. Edited by Philip J. Currie, Eva B. Koppelhus, Martin A. Shugar, and Joanna L. Wright, 215–233. Bloomington, Indiana: Indiana University Press.
Varricchio, David J., and Frankie D. Jackson. 2004b. “A Phylogenetic Assessment of Prismatic Dinosaur Eggs From the Cretaceous Two Medicine Formation of Montana.” Journal of Vertebrate Paleontology 24, no. 4: 931–937.
Varricchio, David J., and Frankie D. Jackson. 2016. “Reproduction in Mesozoic Birds and Evolution of the Modern Avian Reproductive Mode.” The Auk 133, no. 4 (1 October): 654–684.
Varricchio, David J., Jason R. Moore, Gregory M. Erickson, Mark A. Norell, Frankie D. Jackson, and John J. Borkowski. 2008. “Avian Paternal Care Had Dinosaur Origin.” Science 322, no. 5909 (19 December): 1826–1828.
Varricchio, David J., Frankie D. Jackson, Robert A. Jackson, and Darla K. Zelenitsky. 2013. “Porosity and Water Vapor Conductance of Two Troodon formosus Eggs: An Assessment of Incubation Strategy in a Maniraptoran Dinosaur.” Paleobiology 39, no. 2 (8 March): 278–296.
Varricchio, David J., Martin Kundrát, and Jason Hogan. 2018. “An Intermediate Incubation Period and Primitive Brooding in a Theropod Dinosaur.” Scientific Reports 8, no. 1 (August 20): 12454.
Vazquez, Rick J. 1992. “Functional Osteology of the Avian Wrist and the Evolution of Flapping Flight.” Journal of Morphology 211, no. 3 (March): 259–268.
Vickers-Rich, Patricia, Luis M. Chiappe, and Sergei Kurzanov. 2002. “The Enigmatic Birdlike Dinosaur Avimimus portentosus: Comments and a Pictorial Atlas.” In Mesozoic Birds: Above the Heads of Dinosaurs, 65–86. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 65–86. Berkeley, California: University of California Press.
Wang, Min, Zhiheng Li, and Zhonghe Zhou. 2017. “Insight Into the Growth Pattern and Bone Fusion of Basal Birds From an Early Cretaceous Enantiornithine Bird.” Proceedings of the National Academy of Sciences 114, no. 43 (October 9): 11470–11475.
Wang, Min, and Graeme T. Lloyd. 2016. “Rates of Morphological Evolution are Heterogeneous in Early Cretaceous Birds.” Proceedings of the Royal Society B 283, no. 1828 (13 April): 20160214.
Wang, Min, Thomas A. Stidham, and Zhonghe Zhou. 2018. “A New Clade of Basal Early Cretaceous Pygostylian Birds and Developmental Plasticity of the Avian Shoulder Girdle.” Proceedings of the National Academy of Sciences 115, no. 42 (September 24): 10708–10713.
Wang, Min, Xiaoli Wang, Yan Wang, and Zhonghe Zhou. 2016. “A New Basal Bird From China With Implications For Morphological Diversity in Early Birds.” Scientific Reports 6 (25 January): 19700. doi: 10.1038/srep19700.
Wang, Wei, and Jingmai K. O’Connor. 2017. “Morphological Coevolution of the Pygostyle and Tail Feathers in Early Cretaceous Birds.” Vertebrata PalAsiatica 55, no. 4: 289–314.
Wang, Xiaoli, Jingmai K. O’Connor, John N. Maina, Yanhong Pan, Min Wang, Yan Wang, Xiaoting Zheng, and Zhonghe Zhou. 2018. “Archaeorhynchus Preserving Significant Soft Tissue Including Probable Fossilized Lungs.” Proceedings of the National Academy of Sciences 115, no. 45 (April 10): 11555–11560.
Wang, Min, Jingmai K. O’Connor, Yanhong Pan, and Zhonghe Zhou. 2017d. “A Bizarre Early Cretaceous Enantiornithine Bird With Unique Crural Feathers and an Ornithuromorph Plough-Shaped Pygostyle.” Nature Communication 8, no. 1 (January): 14141.
Wang, Min, Jingmai K. O’Connor, Xing Xu, and Zhonghe Zhou. 2019. “A New Jurassic Scansoriopterygid and the Loss of Membranous Wings in Theropod Dinosaurs.” Nature 569, no. 7755 (8 May): 256–259.
Wang, Min, Xiaoting Zheng, Jingmai K. O’Connor, Graeme T. Lloyd, Xiaoli Wang, Yan Wang, Xiamei Zhang, and Zhonge Zhou. 2015. “The Oldest Record of Ornithuromorpha From the Early Cretaceous of China.” Nature Communications 6 (5 May): 6987. doi: 10.1038/ncomms7987.
Wang, Min, and Zhonghe Zhou. 2016. “A New Adult Specimen of the Basalmost Ornithuromorph Bird Archaeorhynchus spathula (Aves: Ornithuromorpha) and Its Implications For Early Avian Ontogeny.” Journal of Systematic Palaeontology 15, no. 1 (3 February): 1–18.
Wang, Min, and Zhonghe Zhou. 2017. “The Evolution of Birds With Implications From New Fossil Evidence.” In The Biology of the Avian Respiratory System. Edited by John N. Maina, 1–26. Berlin, Germany: Springer.
Wang, Shuo, Josef Stiegler, Ping Wu, Ching-Ming Chuong, Dongyu Hu, Amy Balanoff, Yachun Zhou, and Xing Xu. 2017a. “Heterochronic Truncation of Odontogenesis in Theropod Dinosaurs Provides Insight Into the Macroevolution of Avian Beaks.” Proceedings of the National Academy of Sciences 114, no. 41 (September 25): 10930–10935.
Wang, Xiaoli, Jingmai K. O’Connor, Xiaoting Zheng, Min Wang, Han Hu, and Zhonghe Zhou. 2014. “Insights Into the Evolution of Rachis Dominated Tail Feathers From a New Basal Enantiornithine (Aves: Ornithothoraces).” Biological Journal of the Linnean Society 113, no. 3 (November): 805–819.
Wang, Xiaoli, Michael Pittman, Xiaoting Zheng, Thomas G. Kaye, Amanda R. Falk, Scott A. Hartman, and Xing Xu. 2017b. “Basal Baboon Functional Anatomy Illuminated By High-Detail Body Outline.” Nature Communications 8 (March 1): 14576. doi: 10.1038/ncomms14576.
Wang, Xia, Zihui Zhang, Chunling Gao, Lianhai Hou, Qingjin Meng, and Jingyuan Liu. 2010. “A New Enantiornithine Bird from the Early Cretaceous of Western Liaoning, China.” The Condor 112, no. 3 (1 August): 432–437.
Wang, Yan, Han Hu, Jingmai K. O’Connor, Min Wang, Xing Xu, Zhonghe Zhou, Xiaoli Wang, and Xiaoting Zheng. 2017c. “A Previously Undescribed Specimen Reveals New Information On the Dentition of Sapeornis chaoyangensis.” Cretaceous Research 74 (June): 1–10.
Wellnhofer, Peter. 1999. “Atmung bei Dinosauriern und Urvögeln.” Naturwissenschaftliche Rundschau 52, 236.
Wellnhofer, P. 2000. “Hornschnabel bei Confuciusornis.” Naturwissenschaftliche Rundschau 53: 37–38.
Wellnhofer, P. 2002. “Die Gefiederten Dinosaurier von China.” Naturwissenschaftliche Rundschau 55: 465–477.
Wellnhofer, Peter. 2009. Archaeopteryx: The Icon of Evolution. Translated Frank Haase. Munich, Germany: Pfeil.
Widenmeyer, Markus, and Reinhard Junker. 2021. Schöpfung ohne Schöpfer? Eine Verteidigung des Design-Arguments in der Biologie. Holzgerlingen, Germany: SCM Hänssler.
Wiemann, Jasmina, Tzu-Ruei Yang, and Mark A. Norell. 2018. “Dinosaur Egg Color Had a Single Evolutionary Origin.” Nature 563 (31 October): 555–558.
Witmer, Lawrence M. 2002. “The Debate on Avian Ancestry: Phylogeny, Function, and Fossils.” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 3–30. Berkeley, California: University of California Press.
Xu, Xing. 2006. “Feathered Dinosaurs from China and the Evolution of Major Avian Characters.” Integrative Zoology 1, no. 1 (March): 4–11.
Xu, Xing, Yennien Cheng, Xiaolin Wang, and Chunhsiang Chang. 2003a. “Pygostyle-like Structure From Beipiaosaurus (Theropoda, Therizinosauroidea) From the Lower Cretaceous Yixian Formation of Liaoning, China.” Acta Geologica Sinica 77, no. 3 (September): 294–298.
Xu, Xing, Yen-Nien Cheng, Xiaolin Wang, and Chun-Hsiang Chang. 2002a. “An Unusual Oviraptorosaurian Dinosaur From China.” Nature 419, no. 6904 (October): 291–293.
Xu Xing, Jonah Choiniere, Qingwei Tan, Roger B. J. Benson, James Clark, Corwin Sullivan, Qi Zhao, Fenglu Han, Qingyu Ma, Yiming He, Shuo Wang, Hai Xing, and Lin Tan. 2018. “Two Early Cretaceous Fossils Document Transitional Stages in Alvarezsaurian Dinosaur Evolution.” Current Biology 28, no. 17 (10 September): 2853–2860.
Xu, Xing, James M. Clark, Jinyou Mo, Jonah Choinier, Catherine A. Forster, Gregory M. Erickson, David W. E. Hone, et al. 2013. “A Jurassic Ceratosaur From China Helps Clarify Avian Digital Homologies.” Nature 459 (18 June): 940–944.
Xu, Xing, Philip Currie, Michael Pittman, Lida Xing, Qingjin Meng, Junchang Lü, Dongyu Hu, and Congyu Yu. 2017. “Mosaic Evolution in an Asymmetrically Feathered Troodontid Dinosaur With Transitional Features.” Nature Communications 8 (2 May): 14972. doi: 10.1038/ncomms14972.
Xu, Xing, Fenglu Han, and Qi Zhao. 2014. “Homologies and Homeotic Transformation of the Theropod ‘Semilunate’ Carpal.” Scientific Reports 4 (13 August): 6042. doi: 10.1038/srep06042.
Xu, Xing, Mark A. Norell, Xiaolin Wang, Peter J. Makovicky, and Xiao-Chun Wu. 2002b. “A Basal Troodontid From the Early Cretaceous of China.” Nature 415, no. 6873 (March): 780–784.
Xu, Xing, and Diego Pol. 2013. “Archaeopteryx, Paravian Phylogenetic Analyses, and the Use of Probability-Based Methods for Palaeontological Datasets.” Journal of Systematic Paleontology 12, no. 3 (May): 323–334.
Xu, Xing, Qi Zhao, Mark Norell, Corwin Sullivan, David Hone, Gregory Erickson, Xiaolin Wang, Fenglu Han, and Yu Guo. 2009. “A New Feathered Maniraptoran Dinosaur Fossil That Fills a Morphological Gap in Avian Origin.” Chinese Science Bulletin 54, no. 3 (16 December): 430–435.
Xu, Xing, Xiaoting Zheng, and Hailu You. 2009. “A New Feather Type in a Nonavian Theropod and the Early Evolution of Feathers.” Proceedings of the National Academy of Sciences 106, no. 3 (January 20): 832–834.
Xu, Xing, Xiaoting Zheng, Corwin Sullivan, Xiaoli Wang, Lida Xing, Yan Wang, Xiaomei Zhang, Jingmai K. O’Connor, Fucheng Zhang, and Yanhong Pan. 2015. “A Bizarre Jurassic Maniraptoran Theropod With Preserved Evidence of Membranous Wings.” Nature 521, no. 7550 (7 May): 70–73.
Xu, Xing, Xiaoting Zheng, and Hailu You. 2010. “Exceptional Dinosaur Fossils Show Ontogenetic Development of Early Feathers.” Nature 464, no. 7293 (29 April): 1338–1341.
Xu, Xing, Zhonghe Zhou, Robert Dudley, Susan Mackem, Cheng-Ming Chuong, Gregory M. Erickson, and David J. Varricchio. 2014. “An Integrative Approach to Understanding Bird Origins.” Science 346, no. 6215 (12 December): 1341, 1253293-1–1253293-10.
Xu, Xing, Zhonghe Zhou, Xiaolin Wang, Xuewen Kuang, Fucheng Zhang, and Xiangke Du. 2003b. “Four-Winged Dinosaurs from China.” Nature 421, no. 6921 (23 January): 335–340.
Zanno, Lindsay E. 2010. “A Taxonomic and Phylogenetic Re-evaluation of Therizinosauria (Dinosauria: Maniraptora).” Journal of Systematic Palaeontology 8, no. 4 (December): 503–543.
Zelenitsky, Darla Karen. 2006. “Reproductive Traits of Non-Avian Theropods.” Journal of the Paleontological Society in Korea 22, no. 1 (January): 209–216.
Zelenitsky, Darla K, and François Therrien. 2008. “Phylogenetic Analysis of Reproductive Traits of Maniraptoran Theropods and Its Implications For Egg Parataxonomy.” Palaeontology 51, no. 4 (July): 807–816.
Zhang, Fucheng, and Zhonghe Zhou. 2000. “A Primitive Enantiornithine Bird and the Origin of Feathers.” Science 290, no. 5498 (December 8): 1955–1959.
Zhang, Fucheng, Zhonghe Zhou, Lianhai Hou, and Gang Gu. 2001. “Early Diversification of Birds: Evidence From a New Opposite Bird.” Chinese Science Bulletin 46 (June): 945–949.
Zhang, Fucheng, Zhonghe Zhou, Xing Xu, Xiaolin Wang, and Corwin Sullivan. 2008. “A Bizarre Jurassic Maniraptoran From China With Elongate Ribbon-like Feathers.” Nature 455, no. 7216 (23 October): 1105–1108.
Zhang, Zihui, Defeng Cheng, Huitao Zhang, and Lianhai Hou. 2014. “A Large Enantiornithine Bird From the Lower Cretaceous of China and Its Implication For Lung Ventilation.” Biological Journal of the Linnean Society 113, no. 3 (November): 820–827.
Zheng, Xiaoting, Larry D. Martin, Zhonghe Zhou, David A. Burnham, Fucheng Zhang, and Desui Miao. 2011. “Fossil Evidence of Avian Crops From the Early Cretaceous of China.” Proceedings of the National Academy of Sciences 108, no. 38 (September 6): 15904–15908.
Zheng, Xiaoting, Jingmai K. O’Connor, Fritz Huchzermeyer, Xiaoli Wang, Yan Wang, Min Wang, and Zhonghe Zhou. 2013. “Preservation of Ovarian Follicles Reveals Early Evolution of Avian Reproductive Behavior.” Nature 495, no. 7442 (March 28): 507–511.
Zheng, Xiaoting, Jingmai K. O’Connor, Fritz Huchzermeyer, Xiaoli Wang, Yan Wang, Xiaomei Zhang, and Zhonghe Zhou. 2014a. “New Specimens of Yanornis Indicate a Piscivorous Diet and Modern Alimentary Canal.” PLoS ONE 9, no. 4 (April 14): e95036.
Zheng, Xiaoting, Jingmai K. O’Connor, Xiaoli Wang, Min Wang, Xiaomei Zhang, and Zhonghe Zhou. 2014b. “On the Absence of Sternal Elements in Anchiornis (Paraves) and Sapeornis (Aves) and the Complex Early Evolution of the Avian Sternum.” Proceedings of the National Academy of Sciences 111, no. 38 (September 8): 13900–13905.
Zheng, Xiaoting, Jingmai K. O’Connor, Xiaoli Wang, Yanhong Pan, Yan Wang, Min Wang, and Zhonghe Zhou. 2017. “Exceptional Preservation of Soft Tissue in a New Specimen of Eoconfuciusornis and its Biological Implications.” National Science Review 4, no. 3 (May): 441–452.
Zheng, Xiaoting, Xiaoli Wang, Jingmai K. O’Connor, and Zhonghe Zhou. 2012. “Insight Into the Early Evolution of the Avian Sternum From Juvenile Enantiornithines.” Nature Communications 3 (9 October): 1116.
Zhou, Shuang, Zhong-He Zhou, and Jingmai K. O’Connor. 2012. “A New Basal Beaked Ornithurine Bird From the Lower Cretaceous of Western Liaoning, China.” Vertebrata PalAsiatica 50, no. 1 (15 March): 9–24.
Zhou, Yachun, Corwin Sullivan, and Fu-Cheng Zhang. 2019. “Negligible Effect of Tooth Reduction on Body Mass in Mesozoic Birds.” Vertebrata PalAsiatica 57, no. 1 (January): 38–50.
Zhou, Zhonghe. 1995. “Is Mononykus a Bird?” The Auk 112, no. 4 (October): 958–963.
Zhou, Zhonghe. 2004. “The Origin and Early Evolution of Birds: Discoveries, Disputes and Perspectives From Fossil Evidence.” Naturwissenschaften 91 (8 September): 455–471.
Zhou, Zhonghe, and Lianhai Hou. 2002. “The Discovery and Study of Mesozoic Birds in China.” In Mesozoic Birds: Above the Heads of Dinosaurs. Edited by Luis M. Chiappe, and Lawrence M. Witmer, 160–183. Berkeley, California: University of California Press.
Zhou, Zhonghe, Xiaolin Wang, Fucheng Zhang, and Xing Xu. 2000. “Important Features of Caudipteryx—Evidence From Two Nearly Complete New Specimens.” Vertebrata PalAsiatica 38, no. 4 (January): 241–254.
Zhou, Zhonghe, and Fucheng Zhang. 2002. “A Long-Tailed, Seed-Eating Bird From the Early Cretaceous of China.” Nature 418, no. 6896 (25 July): 405–409.
Zhou, Zhonghe, and Fucheng Zhang. 2003a. “Jeholornis Compared to Archaeopteryx, With a New Understanding of the Earliest Avian Evolution.” Naturwissenschaften 90, no. 5 (May): 220–225.
Zhou, Zhonghe, and Fucheng Zhang. 2003b. “Anatomy of the Primitive Bird Sapeornis chaoyangensis From the Early Cretaceous of Liaoning, China.” Canadian Journal of Earth Sciences 40 (May 21): 731–747.
Zhou, Zhonghe, and Fucheng Zhang. 2005. “Discovery of an Ornithurine Bird and its Implication For Early Cretaceous Avian Radiation.” Proceedings of the National Academy of Sciences 102, no. 52 (December 12): 18998–19002.
Zhou, Zhonghe, and Fucheng Zhang. 2006a. “Mesozoic Birds of China—A Synoptic Review.” Vertebrata PalAsiatica 44, no. 1 (15 March): 74–98.
Zhou, Zhonghe, and Fucheng Zhang. 2006b. “A Beaked Basal Ornithurine Bird (Aves, Ornithurae) From the Lower Cretaceous of China.” Zoologica Scripta 35, no. 4 (July): 363–373.
Zimbelmann F. 1999. “Sind Vögel Dinosaurier mit Federn?” Studium Integrale Journal 6, no. 1 (March): 3–7.












